Engineers from the University of Delaware (UD) have found a method to effectively capture 99% of carbon dioxide from air using a unique electrochemical system run with hydrogen.
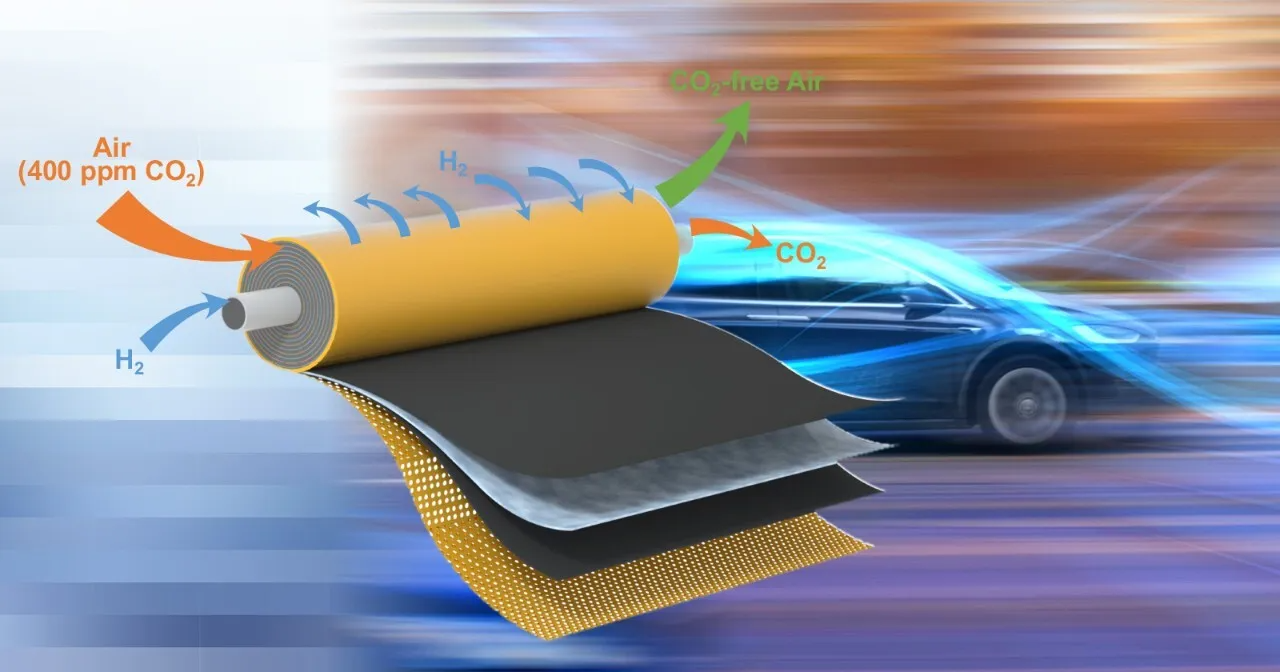 University of Delaware researchers have broken new ground that could bring more environmentally friendly fuel cells closer to commercialization. Image Credit: Spiral module schematic provided by the Yan lab.
University of Delaware researchers have broken new ground that could bring more environmentally friendly fuel cells closer to commercialization. Image Credit: Spiral module schematic provided by the Yan lab.
It is a major progress for carbon dioxide capture and could pave the way toward more commercially eco-friendly fuel cells. The research team, headed by UD Professor Yushan Yan, explained their technique in Nature Energy (February 3rd issue).
Game-Changing Tech for Fuel Cell Efficiency
Fuel cells operate by turning fuel chemical energy straight into electricity. They can be utilized in the transportation sector for hybrid or zero-emission vehicles.
Yan, Henry Belin du Pont Chair of Chemical and Biomolecular Engineering, has been involved for quite a while in the task of enhancing hydroxide exchange membrane (HEM) fuel cells, a cost-effective and environmentally friendly substitute to traditional acid-based fuel cells employed at present.
But HEM fuel cells have a limitation that has hindered their progress—they are highly sensitive to carbon dioxide in the air. In simpler terms, the carbon dioxide obstructs a HEM fuel cell from breathing.
This flaw quickly decreases the fuel cell’s efficiency and performance by nearly 20%, rendering the fuel cell the same as a gasoline engine. Yan’s research team has been hunting for a workaround for this carbon dioxide challenge for more than 15 years.
During the past few years, the scientists understood this disadvantage might actually be a solution—for the elimination of carbon dioxide.
Once we dug into the mechanism, we realized the fuel cells were capturing just about every bit of carbon dioxide that came into them, and they were really good at separating it to the other side.
Brian Setzler, Study Lead Author and Assistant Professor for Research in Chemical and Biomolecular Engineering, University of Delaware
While this is not feasible for the fuel cell, the researchers knew if they could exploit this integral “self-purging” process in a separate device upstream from the fuel cell stack, they could transform it into a carbon dioxide separator.
“It turns out our approach is very effective. We can capture 99% of the carbon dioxide out of the air in one pass if we have the right design and right configuration,” said Yan.
So, how was this achieved?
They discovered a way to implant the power source for the electrochemical technology inside the separation membrane. The method required internally short-circuiting the device.
It's risky, but we managed to control this short-circuited fuel cell by hydrogen. And by using this internal electrically shorted membrane, we were able to get rid of the bulky components, such as bipolar plates, current collectors or any electrical wires typically found in a fuel cell stack.
Lin Shi, Study Lead Author and Doctoral Candidate, Yan group
This provided the research team with an electrochemical device that resembled a standard filtration membrane manufactured for separating out gases, but with the capability to nonstop pick up small amounts of carbon dioxide from the air like a more complex electrochemical system.
In short, implanting the device’s wires within the membrane formed a shortcut that made it easier for the carbon dioxide particles to move from one side to the other. It also allowed the team to build a compact, spiral module possessing a large surface area in a small volume.
In other words, they currently have a smaller package that can filter greater quantities of air at a time, making it effective as well as economical for fuel cell applications. Meanwhile, fewer parts mean less cost and, more notably, provided a means to effortlessly scale up for the market.
The research team’s outcomes revealed that an electrochemical cell measuring 2 inches by 2 inches could continually eliminate around 99% of the carbon dioxide found in the air flowing at a rate of roughly two liters per minute.
An early spiral device model roughly the size of a 12-ounce soda can is capable of filtering 10 liters of air per minute and eliminating 98% of the carbon dioxide, the scientists explained.
Designed for an automotive application, the device would approximately be the size of a gallon of milk, Setzer described, but the device could be employed to eliminate carbon dioxide elsewhere, too. For example, the UD-patented technology could allow lighter, more efficient carbon dioxide elimination devices in submarines or spacecraft, where continuous filtration is very important.
We have some ideas for a long-term roadmap that can really help us get there.
Brian Setzler, Study Lead Author and Assistant Professor for Research in Chemical and Biomolecular Engineering, University of Delaware
According to Shi, since the electrochemical device is driven by hydrogen, as the hydrogen economy advances, this electrochemical device could also be employed in buildings and airplanes where air recirculation is preferred as an energy-saving measure.
After his dissertation defense, Shi will collaborate with Versogen, a UD spinoff company started by Yan, to continue progressing research toward sustainable green hydrogen.
Co-authors on the article from the Yan lab include Yun Zhao, co-first author and research associate, who carried out experimental work crucial for testing the device, Stephanie Matz, a doctoral student who handled the designing and fabrication of the spiral module, and Shimshon Gottesfeld, an adjunct professor of chemical and biomolecular engineering at UD.
Gottesfeld was the principal investigator on the 2019 project that was financially backed by the Advanced Research Projects Agency-Energy (ARPA-E), which led to the findings.
Journal Reference:
Shi, L., et al. (2022) A shorted membrane electrochemical cell powered by hydrogen to remove CO2 from the air feed of hydroxide exchange membrane fuel cells. Nature Energy. doi.org/10.6084/m9.figshare.16744258.