A collaborative study headed by Professor Xiujie Wang at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences, Professor Charlie Wang C. L. at The University of Manchester, and Professor Yongjin Liu at the TsingHua University, has announced a novel three-dimensional (3D) bioprinting platform.
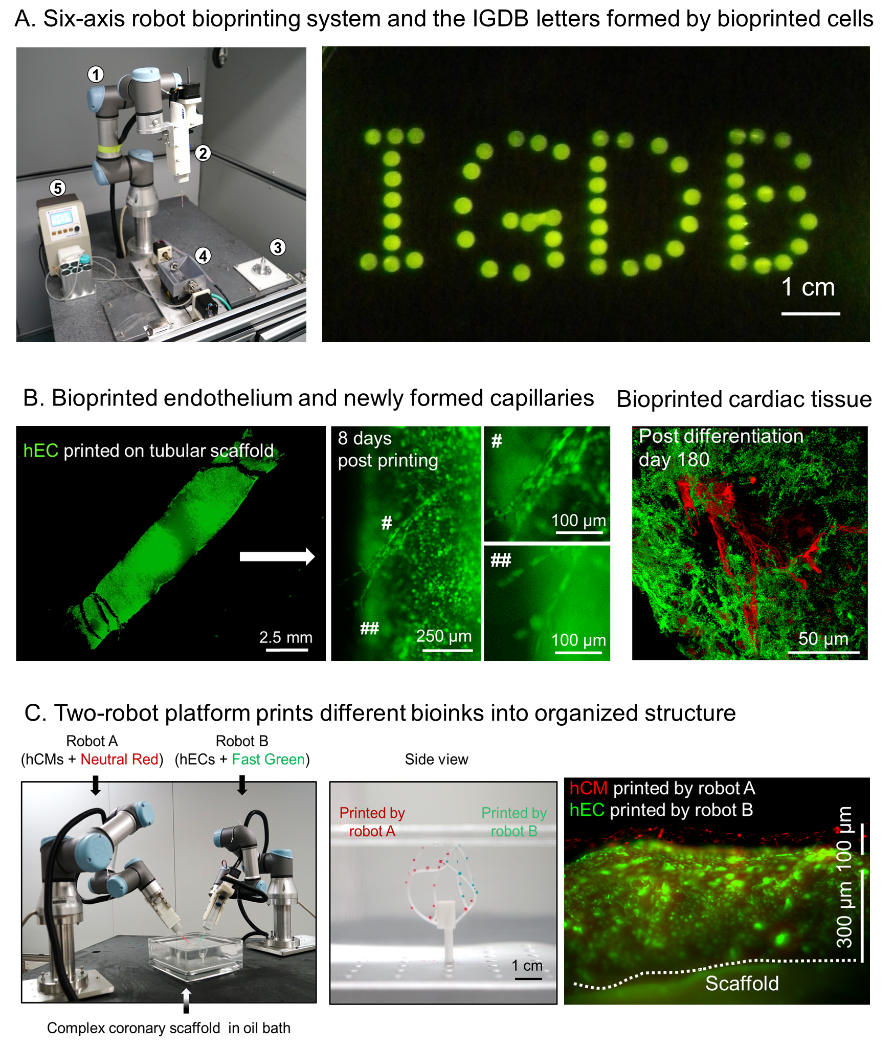 Novel six-axis robot-based bioprinting system and its printing products. A. Six-axis robot bioprinting platform and the printed IGDB letters composed of eGFP-labeled endothelial cells; B. Bioprinted artificial blood vessel is capable of forming new capillaries (left and middle, green color is eGFP-labeled endothelial cells). Bioprinted vascularized cardiac tissue (right, green color is cardiac tissue and red color is vascular network); C. Two-robot cooperation platform (left) can simultaneously print different types of cells onto a complex-shaped vascular scaffold (middle) to form patterned cell organization (right). Image Credit: Institute of Genetics and Developmental Biology.
Novel six-axis robot-based bioprinting system and its printing products. A. Six-axis robot bioprinting platform and the printed IGDB letters composed of eGFP-labeled endothelial cells; B. Bioprinted artificial blood vessel is capable of forming new capillaries (left and middle, green color is eGFP-labeled endothelial cells). Bioprinted vascularized cardiac tissue (right, green color is cardiac tissue and red color is vascular network); C. Two-robot cooperation platform (left) can simultaneously print different types of cells onto a complex-shaped vascular scaffold (middle) to form patterned cell organization (right). Image Credit: Institute of Genetics and Developmental Biology.
3D bioprinting, which integrates a 3D printer with bioinks (frequently consisting of biomaterials and cells) to fabricate tissue- or organ-mimicking structures, is one of the most hopeful technologies for in vitro human organ generation.
But the commonly utilized 3D bioprinting method is incapable of integrating blood vessel networks at the time of the bioprinting process and thus experiences difficulty when fabricating functional and long-lived complex organs as a result of the lack of nutrient supply to printed cells.
Present bioprinting technologies depend on immobilizing printed cells by adding artificial biomaterials to the bioinks. This hinders functional cell contact and the development of new blood vessels, thereby interfering with the biological function and long-lasting survival of bioprinting products.
In this study, the scientists creatively turned a six-axis robotic arm into a 3D bio-printer, thereby allowing cell printing from all directions. To prevent the harmful effects of biomaterial solidification, they also fabricated an oil-bath-based cell printing system that enables the printed cells to blood vessel scaffolds through hydrophobicity. This helps in better maintaining cell activity and encouraging the formation of cell-to-cell contact.
Integrating the six-axis robot-based bioprinter and oil-bath-based cell-printing system, the scientists obtained a complete range of cell printing on a complex-shaped blood vessel scaffold without damaging cells or compromising cell function and proliferation.
Moreover, on gaining inspiration from the natural organ developmental process, the team developed a repeated print-and-culture bioprinting strategy: after printing mono- or multi-layers of cells on the blood vessel scaffold, the printed cells would be cultured for specific intervals to induce the formation of cell-cell contact and new blood vessels. Further, the scaffold and already printed cells were exposed to a new round of bioprinting.
In theory, repetition of such a print-and-culture cycle could produce complex tissue or organs with printed cells interlaced and linked with blood vessel networks to retain long-term survival and functions.
To illustrate the possibility of such a strategy, the scientists performed bioprinting experiments with the help of endothelial cell bioink and cardiomyocyte bioink on blood vessel scaffolds and discovered that the printed endothelial cells developed intact endothelium as well as new blood vessels and capillaries with the help of angiogenic factors.
At the same time, the printed cardiomyocytes developed gap junctions and recovered rhythmic contraction shortly following printing.
Through printing the mixture of endothelial cells and cardiomyocytes, scientists produced a piece of vascularized cardiac tissue, retaining rhythmic beating and alive for a minimum of 6 months.
Taking advantage of the extendibility of the six-axis robots, they additionally established a two-robot cooperation platform and accomplished concurrent bioprinting of multiple types of cells on complex-shaped blood vessel scaffolds.
In summary, compared with traditional approaches, the novel 3D bioprinting system reported in this study provides a new strategy to print cells on complex-shaped vascular scaffolds and streamline angiogenesis amongst post-printed cells.
Thus, it allows long-term cell survival and illustrates a possible manner to produce large-scale and functional artificial tissues or organs in vitro.
The study was published on February 19th, 2022, in the journal Bioactive Materials.
This study was financially supported by grants from the CAS Strategic Priority Program and the National Natural Science Foundation of China.
Journal Reference:
Zhang, Z., et al. (2022) A multi-axis robot-based bioprinting system supporting natural cell function preservation and cardiac tissue fabrication. Bioactive Materials. doi.org/10.1016/j.bioactmat.2022.02.009.