 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 9 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Mar 9 2022In an article recently published in the open-access journal Materials, researchers discussed the inclusion of optical density into a biocement solution blending design.

Study: Incorporation of Optical Density into the Blending Design for a Biocement Solution. Image Credit: MK photograp55/Shutterstock.com
Background
Carbonate diagenesis is the cementing process of natural sediments by using carbonates. Unconfined compressive strength and calcium carbonate content was found to have significant relationships in naturally deposited marine soils. The microbial precipitation of carbonate via ureolytic activity is becoming more popular around the world, and the scope of research is broadening.
The evaluation of microbial carbonate precipitation can be done with high levels of induced carbonates, whether natural or artificial. For engineering practice, however, it is necessary to forecast or design the eventual amounts of induced carbonates.
There are some major issues associated with using microbial carbonate precipitation to improve soils, rocks, and concrete. Bacteria and media have been injected into soils for in situ culture in a number of experiments. In those instances, bacteria cultivation is not always effectively controlled, and the blended biocement solution (BCS) cannot be designed.
Reliability and reproducibility are challenging to achieve in this situation. Engineering procedures for deploying microbial carbonate precipitation necessitate the creation of a mixing biocement solution (BCS). BCS is commonly mixed with NO-A10 concentrated strains, reaction media like urea and CaCl2, and a solvent like water or seawater. The unknown microbiological features, such as cell viability, make the characterization of BCS complicated.
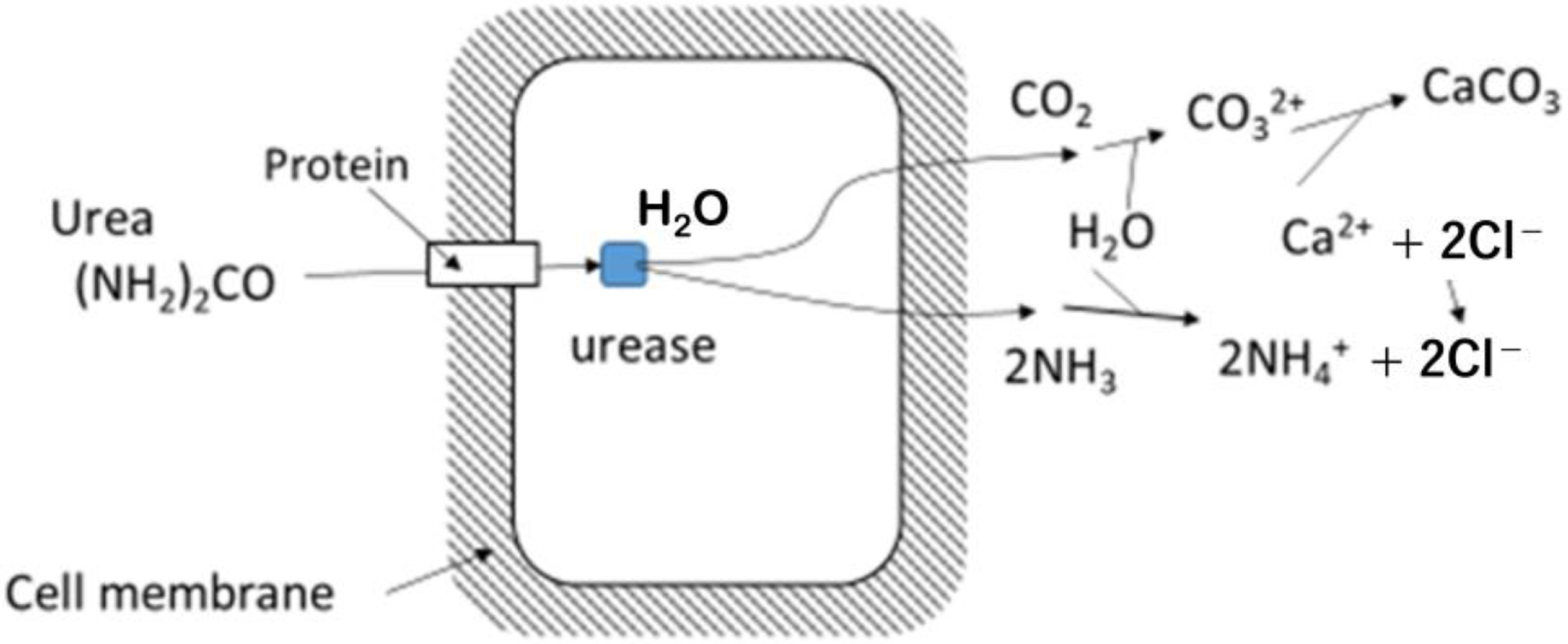
Microbial hydrolysis of urea, showing that first products are CO2 and NH3 inside the microbe’s cell and carbonates are produced outside of the cell. Image Credit: Fukue, M et al., Materials
About the Study
In this study, the authors presented the development of a standard precipitation curve based on the precipitation rate at 24 hours to determine Rcv values. The optical density (OD) was redefined as Rcv OD*, where OD* was the BCS's tentative OD and Rcv was the cell viability conversion rate. The Rcv and OD values of unknown BCS were calculated using arbitrary OD* values and the outcomes of precipitation testing.
The second-order term of OD or OD* was also determined when the testing time was extended. OD was used to identify the best BCS blending.
A basic calibration curve was created based on the notion that CPR was dictated by OD with viable cells. The OD* was linked to the equation OD = Rcv OD*, where Rcv was the conversion factor for cell viability. In terms of Rcv, the CPR-OD* relationship was translated to the CPR-OD relationship. The standard precipitation curve was created in this work, and the blending design of BCS was obtained using 8.46 OD or 8.46 Rcv OD*. This study was primarily focused on the microbial strain (NO-A10).
Stored frozen microorganisms were used to tackle the problem of unpredictable BCS quality. Microbes were grown in a cell incubator and prepared using a continuous supply system. The principles of CPR were investigated in the laboratory using fresh culture.
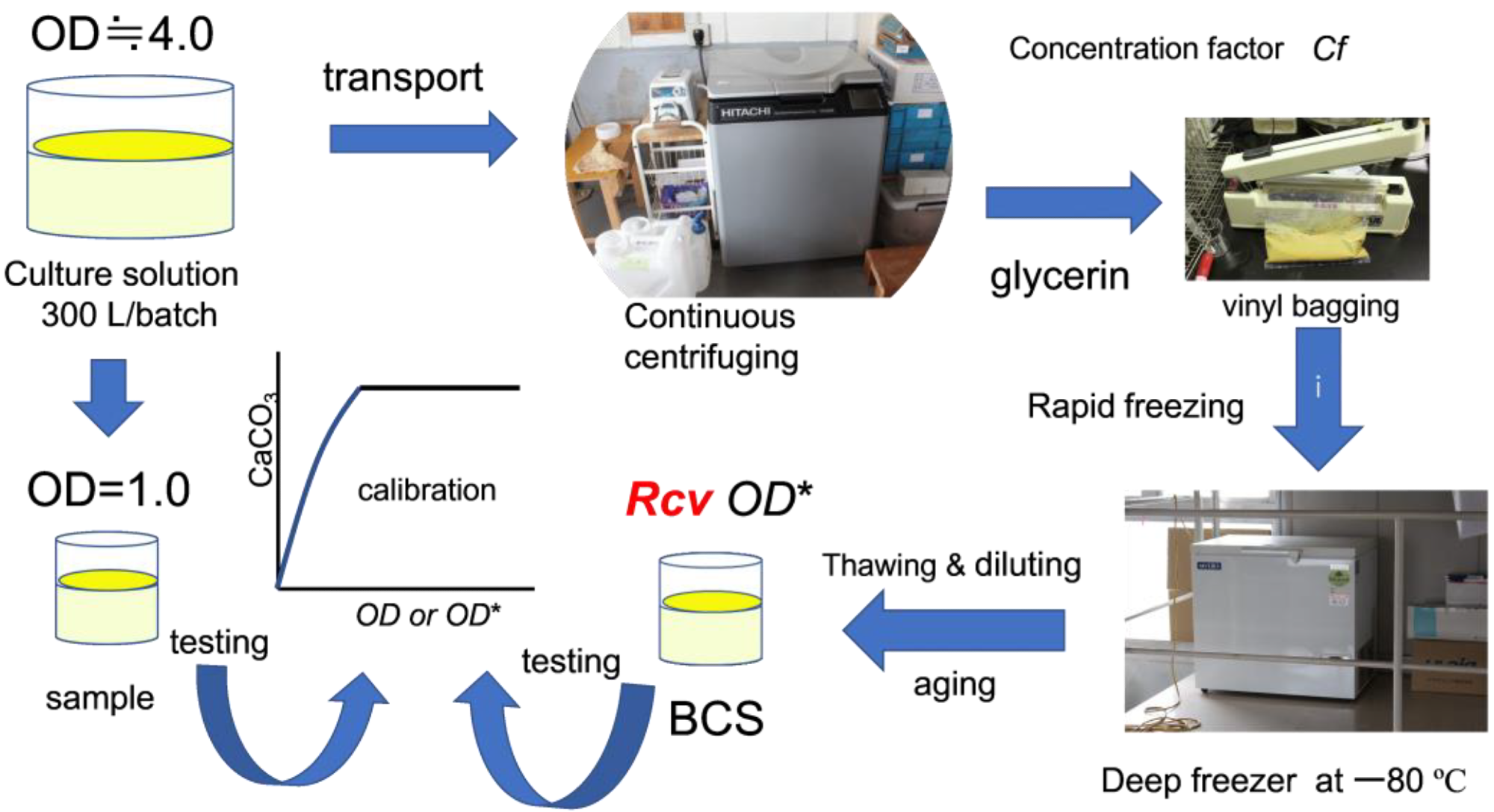
From culture solution to biocement solution. Image Credit: Fukue, M et al., Materials
Observations
The curve was found to be expressed as λ1 OD + λ2 OD2, where λ1 and λ2 were 8.46 M and –17.633 M, respectively. The amount of precipitation was expressed as 8.46 OD, with the OD estimated using precipitation tests with arbitrary OD* values of BCSs. Dilution of the 1.0 OD solution yielded various OD values. Because of the 20 mM ammonium buffer, the initial pH value was around 9.5. When compared to the unique traditional CPR-OD relationship and the experimental relationship of CPR-OD*, it was discovered that Rcv is predictable.
The OD or Ca2+ regulated CPR. The CPR (24 h) was expressed as CPR = 8460 OD - 17.633 OD2 if the OD was dominating. For NO-A10 strains, CPR= a CaCO3 (M/24h) and an M Ca2+ if Ca2+ was dominant. When Ca2+ was high and OD was low, the over-loading effect occurred, which resulted in extremely low CPR. The carbonate precipitation, on the other hand, increased as the notion was extended through time. The relationship of CPR-OD was simply written as CPR = 1 OD, where 1 was 8.46 M for NO-A10 strains.
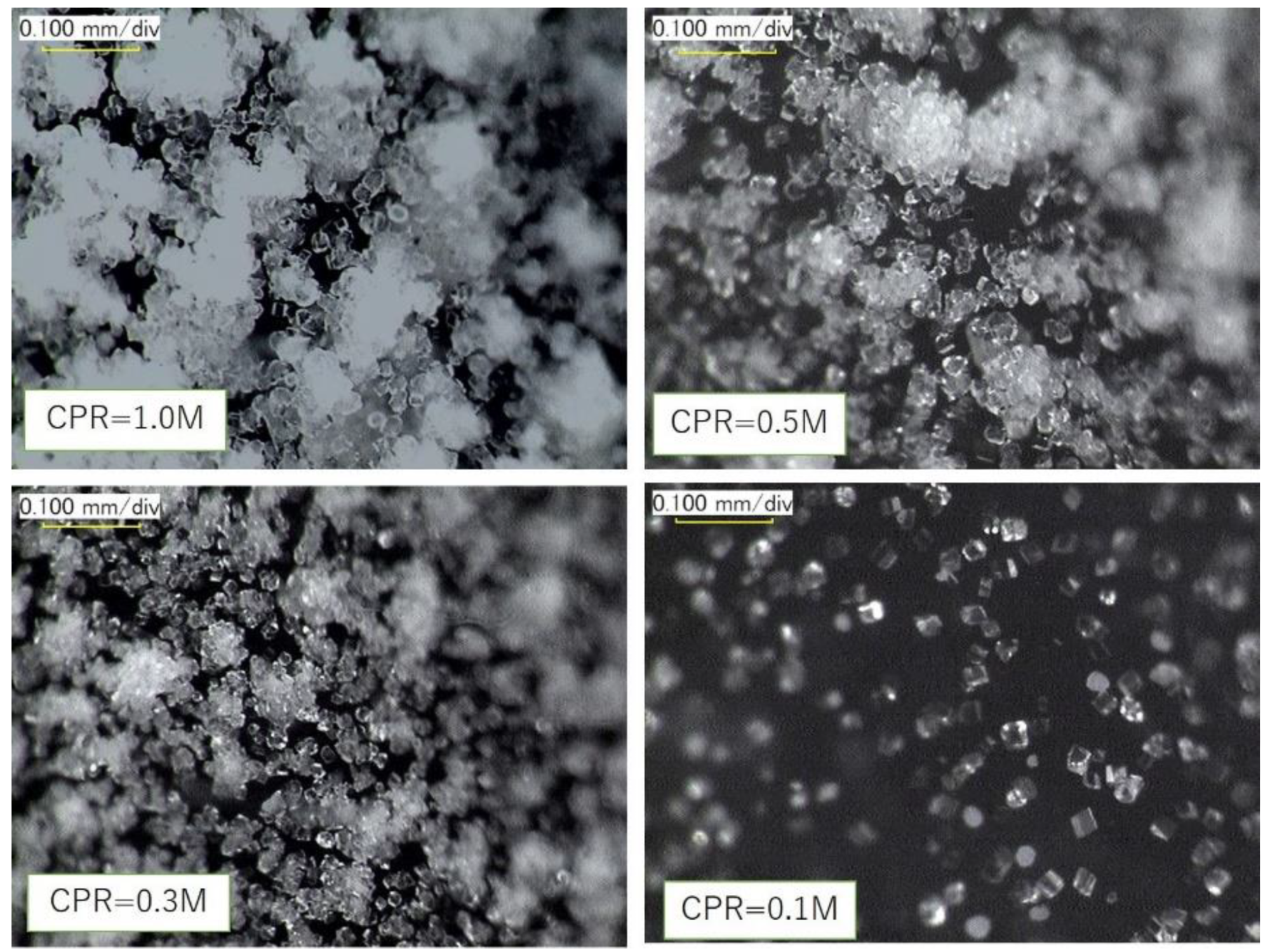
Digital photomicrographs of precipitated CaCO3 (at 24 h) on the walls of test tubes, using various levels of Ca2+. Image Credit: Fukue, M et al., Materials
Conclusions
In conclusion, this study demonstrated that carbonate is precipitated on the glass surfaces like minerals. The Ca2+ and OD* values were influenced by the action of adsorption between microorganisms and soil particles. It was also found to be dependent on the depth of the soil or the distance traveled by the solution as it is injected.
The authors emphasized that future research into the adsorption characteristics and behavior of Ca2+ and microorganisms on soil particles is crucial. They also believe that the discovery that Rcv, OD*, and Ca2+ are all involved in CPR is the most significant contribution of this study.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Fukue, M., Lechowicz, Z., Fujimori, Y., et al. Incorporation of Optical Density into the Blending Design for a Biocement Solution. Materials 15(5), 1951 (2022). https://www.mdpi.com/1996-1944/15/5/1951