Reviewed by Alex SmithApr 11 2022
At the Chinese Academy of Sciences, a recent study was performed by scientists redefining how liquids retain their contact with solid surfaces. It is also called wettability — from an intermolecular force point of view.
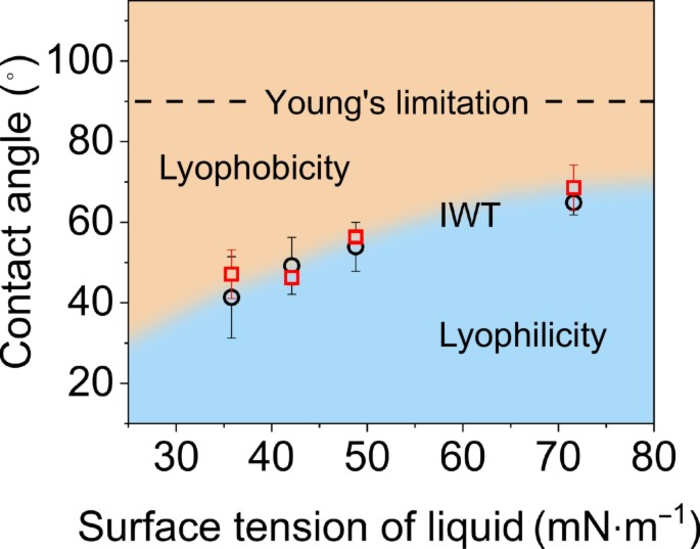 Relationship between surface tension of pure liquids and internal wetting thresholds. Image Credit: Nano Research.
Relationship between surface tension of pure liquids and internal wetting thresholds. Image Credit: Nano Research.
The study findings were published in the journal Nano Research on February 8th, 2022.
Wettability is considered appropriate to the design of materials since it identifies how layers tend to stick together.
Plays a crucial role in many fields, such as the efficiency of catalytic reaction, separation, electrode materials, and the design of bionic smart materials.
Ye Tian, Study Author and Professor, Key Laboratory of Bio-inspired Materials and Interfacial Science, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences
For instance, smart layers that tend to alter their contact based on moisture could be utilized in sportswear that has the potential to adapt to humidity.
Wettability Models
High wettability implies that a liquid drop spreads and results in making a low angle of contact with the surface, whereas low wettability is characteristic of a liquid that hinders spreading. In a classic way, wettability, as denoted by the contact angle, has been defined with the help of Young’s equation. This model has a suitable and perfectly smooth surface.
If the water droplet diffuses to a contact angle below 90°, the surface has been classified as water-loving or hydrophilic. If the water droplet tends to make a contact angle that is higher than 90° degrees, the surface is then categorized as hydrophobic.
But Young’s model has limitations in describing the observed behavior of liquids when it comes in contact with solid surfaces. For instance, it cannot describe why water contact angles increase following the roughness of surfaces, which was explained in a later Wenzel and Cassie model.
Furthermore, the study authors analyzed the interactions of solid surfaces that have been immersed in pure liquids at a molecular level to better comprehend how the intrinsic wetting thresholds (IWTs)—the points at which liquids tend to bead or spread.
A series of studies have found that hydrophobic attraction can exist between apolar surfaces and hydrophilic repulsion between the polar surface(s) in water, that is, the IWTs should depend on the intermolecular forces.
Ye Tian, Study Author and Professor, Key Laboratory of Bio-inspired Materials and Interfacial Science, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences
Intrinsic Wetting Thresholds
The scientists experimented with interactions of solids made up of one-molecule-thick layers (self-assembled monolayers or SAMs) in various liquids to look at how wettability impacted their repulsion or attraction.
Dimethyl sulfoxide (DMSA), ethylene glycol (EG), Water and N, N-dimethyl formamide (DMF) were chosen as the testing liquids to depict a range of surface tensions. With the help of an atomic force microscope, the scientists quantified force curves for the adhesion forces present between the SAMs in each liquid.
Contact angles were evaluated for 1 μL droplets of each liquid with the help of a Contact Angle System. It is a device that has been developed in such a way that it quantifies and analyzes drop shape and contact angle with the solid.
The outcomes displayed that for water, the intrinsic wetting threshold (IWT) took place at a contact angle of 65° with the solid, not the 90° anticipated by Young’s equation. Put differently, 65° was the interface point between hydrophilic and hydrophobic behavior, which has to deal with variations in the hydrogen bond networks of water on either side of the threshold.
Moreover, researchers discovered differences in the adhesion forces between the water layer and the hard surfaces (SAMs) along with the transition at a contact angle of nearly 65°.
We confirmed that the IWT for pure water is about 65° from the view of interaction forces between symmetrical SAMs.
Ye Tian, Study Author and Professor, Key Laboratory of Bio-inspired Materials and Interfacial Science, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences
The other organic liquids have inadequate hydrogen bonds, but still, the IWTs were achieved by looking at variations in adhesion forces between the hard surfaces (SAMs) together with the contact angles.
The outcomes offered “a new curve of the IWTs, as distinct from the value defined by Young’s equation, which can be used to prejudge the IWTs for pure liquids with known surface tensions.”
Next Steps
The scientists plan to keep studying the mechanisms of wetting at a molecular level, providing considerable applications to the design of functional materials. Having redefined the IWTs comparative to Young’s historic equation, they expect to “provide a new perspective to understand the relationships between wettability and intermolecular force,” expects Tian.
The study authors include Yulong Li, Shaofan He, Zhe Xu, Zhonglong Luo, Hongyan Xiao, Ye Tian, and Lei Jiang.
This study was financially supported by the National Natural Science Foundation of China, Frontier Science Foundation, National Research Fund for Fundamental Key Projects, and the China Postdoctoral Science Foundation.
Journal Reference:
Li, Y., et al. (2022) Investigation on the intrinsic wetting thresholds of liquids by measuring the interaction forces of self-assembled monolayers. Nano Research. doi.org/10.1007/s12274-022-4094-z.