A team of scientists has investigated the use of composite graphene oxide/chitosan adsorbents. The new paper, which has been published in the journal Water, provides a comprehensive evaluation of the proposed material’s ability to remove copper cations from wastewater.

Study: Graphene Oxide-Chitosan Composites for Water Treatment from Copper Cations. Image Credit: Joyseulay/Shutterstock.com
Wastewater Treatment
Population growth, rapid urbanization, and heavy industry have caused ecological challenges which must be overcome to improve the sustainability of modern society. A key challenge facing scientists currently is the effective treatment of wastewater from industrial and domestic sources to limit contaminants entering water systems and improve water security for the world’s population.’
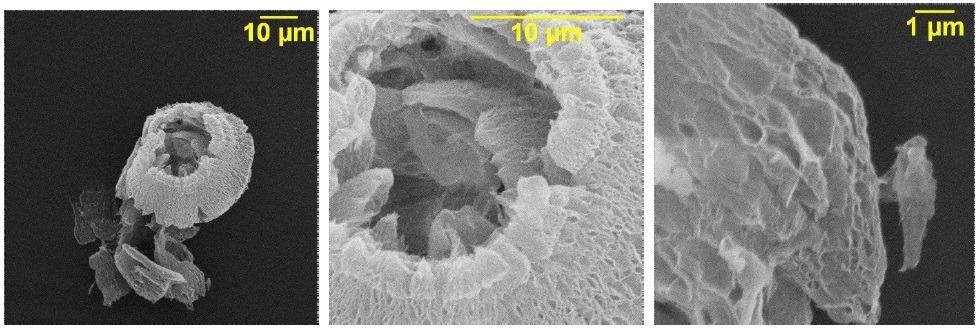
Scanning electron microscopy of thermally reduced graphene oxide. Image Credit: Politaeva, N et al., Water
Several methods have been developed over the years to treat wastewater, including biological, chemical, mechanical, and physical-chemical techniques. Amongst physical-chemical methods, sorbent materials are widely applied to the removal of contaminants such as heavy metals and petroleum products. Additionally, sorbents can be manufactured from waste materials, further improving the sustainability of wastewater treatment.
Carbon-based Sorbents
Two- and three-dimensional carbon-based materials have emerged in recent years as promising candidates for sorbent materials. A common class of materials used for treating wastewater is fullerenes, along with by-products such as soot and niello. Previous studies have demonstrated that a sorbent made from fullerene soot and chitosan can remove 83% of iron cations.
Graphene derivatives have shown particular promise for the removal of pollutants from wastewater. GO has potent antimicrobial properties and have been shown to be as effective as chlorine. Studies have applied the use of GO to remove E. Coli from tap water. Laser-induced graphene has been used to construct membranes for disinfection and purification, and composites of laser-induced graphene and polymers can increase the strength of membranes.
Microscopic GO particles have also been studied for the absorption and removal of radioactive substances. Microscopic GO plates have been used to absorb plutonium and uranium salts, with studies observing that their action is not affected by pH or temperature. The spent GO can then be easily disposed of via incineration. Studies have considered mixing polyethylene with oxidized graphene to improve dispersibility.
Studies have investigated the removal of methylene blue from wastewater using sodium alginate/GO nanocomposites. These have been applied in various forms, such as balls, hydrogels, and fibers. Several other materials have been developed in different studies.
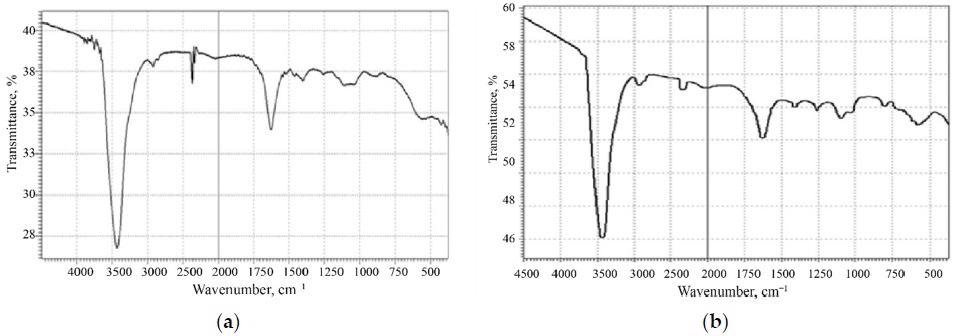
FTIR spectroscopy of: (a) thermally reduced graphene oxide, (b) graphene oxide. Image Credit: Politaeva, N et al., Water
A promising biopolymer matrix for GO composite sorbents is chitosan, which is highly efficient for removing heavy metal ions and radioactive ions from wastewater. Chitosan possesses favorable properties. These include the formation of complexes and chelates, good ion-exchange properties, and unique sorption capabilities. Carbon materials such as nanotubes and graphene improve the thermal, mechanical, and electrical properties of biopolymer matrices.
Chitosan/GO composites have become a focus of several recent studies. The influence of GO on the mechanical and physical properties of chitosan films has been studied, which used IR spectra to confirm the presence of strong interactions between the composite components’ functional groups. These composite sorbents have displayed potent antimicrobial activity against common waterborne pathogens such as P. aeruginosa.
The Paper
The new paper published in Water has investigated the optimal GO percentage in chitosan/GO composite sorbents to improve their mechanical strength and effectiveness. Furthermore, they have evaluated these composite sorbents effectiveness for absorbing and removing copper metal cations, a common heavy metal contaminant of wastewater.
The optimal sorbent content was discovered in the research. A 55.5% GO-44.5% chitosan content by dry granule mass possesses the optimal properties out of the prepared samples. Purification of copper cations in wastewater was extremely high at 96%. The material possesses adequate mechanical properties which make it an attractive candidate for wastewater treatment.
Parameters were studied to elucidate their influence on the composite materials. These were temperature, pH, sorption time, and sorbent dosage. Additionally, the authors demonstrated the material’s optimal capacity to adsorb copper cations by plotting the adsorption isotherm of the materials.
Porous surface characteristics and functional groups were observed in the prepared material using infrared spectroscopy and microstructural studies. Based on these observations, the authors proposed that the process of copper cation absorption is governed by chemisorption and physical sorption processes. Furthermore, the authors determined sorption processes for iodine and methylene blue.
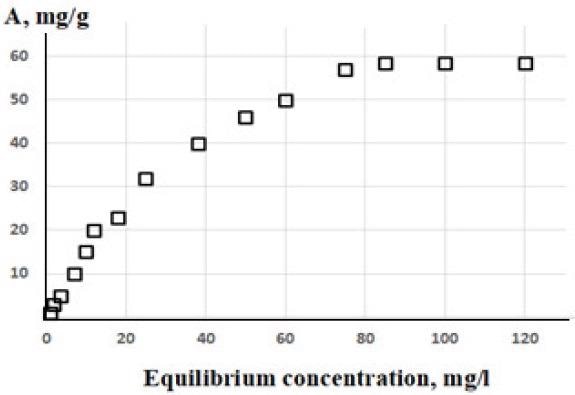
Isotherm of copper cation sorption by GO-Ch composite (sample No. 4). Image Credit: Politaeva, N et al., Water
The authors have proposed using the spent material as a soil additive. This would improve the circularity of the process, removing contaminants from wastewater and improving water security whilst providing a value-added product for the agricultural industry.
Further Reading
Politaeva, N et al. (2022) Graphene Oxide-Chitosan Composites for Water Treatment from Copper Cations Water 14(9) 1430 [online] mdpi.com. Available at: https://www.mdpi.com/2073-4441/14/9/1430
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.