In a paper recently published in the open-access journal Energies, researchers reviewed and presented modern developments in the end-of-life (EOL) photovoltaic modules' (PVMs) recovery methods. They also identified the interconnected observations that accelerated or limited these processes.

Study: End-of-Life Photovoltaic Modules. Image Credit: foxbat/Shutterstock.com
Background
Global energy decarbonization has been receiving a push from renewable energy sources. In 2021, solar energy contributed to more than half of the renewable power expansion. Being the richest as well as the safest energy source on earth, solar energy can be harvested using PVMs.
Harnessing solar energy using photovoltaics (PVs) can be highly scalable, versatile, and attracts a lot of attention from researchers and industries. An increased interest in PVs has dropped their cost by over a factor of 10,000 since their inception, and accelerated adoption of PVs would further reduce their unit costs. However, PVMs have a useful life of only 20 to 35 years. Thus, a majority of the PVMs are expected to reach their EOL by 2050, estimated to be over 78 million tons.
Therefore, the mismanagement of EOL PVMs can result in terrestrial pollution. On the contrary, their successful recovery could reduce the exploitation of resources and waste generation, as well as generate adequate economic returns. This review seeks to provide an overview of the existing EOL PVM recovery methods, recovery of embedded metals in the modules, and the factors that accelerate or impede the responsible and effective circulation strategies for PVs.
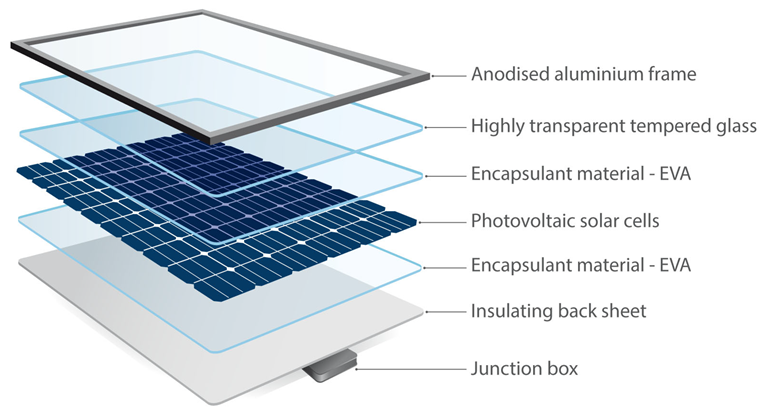
Structure of a photovoltaic module. Reproduced with permission from Global Sustainable Energy Solutions Pty Ltd. (GSES). Image Credit: Tan, J et al., Energies
PVM Design
Due to their outdoor usage, PV cells are packed in between glass sheet layers and flexible polymer encapsulants and then set up on an aluminum frame, protecting them from potentially harsh and changing weather conditions. Inside the PVM, elements like silicon, copper, silver, tin, and lead are embedded. Since these metals are rare, it is necessary to recover and recycle them for new applications. However, their separation is challenging due to the compact and interconnected design of the PVMs.
Recovery Methods for EOL PVMs
The PVM components have to be carefully extracted to retain the purity and the inherent value of the recovered materials. One solution to maximizing the quantity and the value of the recovery is the pre-treatment of the EOL PVMs. These treatment methods are categorized into chemical, physical, and mixed.
The physical recovery methods comprise mechanical methods and pyrolysis. In the mechanical method, the aluminum frame and the junction box are dismantled, while the remnant is crushed and sorted based on its physical characteristics. Pyrolysis involves the recovery of clean PV cells by placing the EOL PVMs in furnaces at temperatures over 400 °C. As there is no crushing involved, the glass and other remnants can be recycled. However, it is an energy-intensive process using high-specification equipment.
The chemical methods include inorganic and organic solvent dissolution. The inorganic dissolution method involves the immersion of the EOL PVM in an alkali or acid solution, which dissolves the ethylene-vinyl acetate (EVA) film and retains the PV cell sheet. Common solvents used are hydrofluoric acid and nitric acid. The organic dissolution method uses the expansion and dissolution of the EVA film to extract the PV cell.
A physical-chemical method combines the aforementioned methods, eliminating their drawbacks and benefitting from their merits. Although this method has better recovery and waste utilization rates, it is energy-intensive, complex, and generates waste liquids.
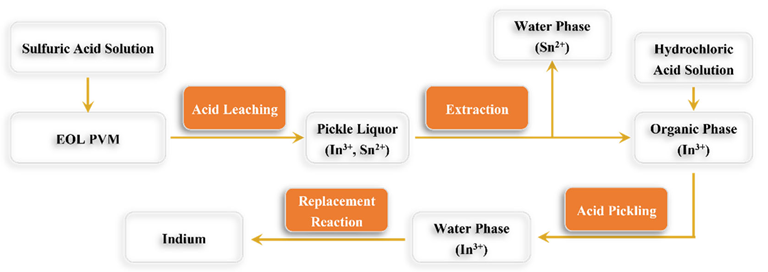
Flow diagram of indium extraction using the acid leaching method. Image Credit: Tan, J et al., Energies
Recovery of Embedded Metals
The valuable metals contained in EOL PVMs can be recovered via solvent extraction, vacuum distillation, and acid leaching. Solvent extraction uses an organic solvent that separates the metal ions by forming a coordination compound with them and then transfers them to other organic solvents. Acid leaching involves leaching the scattered metals from copper indium gallium selenide (CIGS) and cadmium telluride (CdTe) PV cells using concentrated inorganic acids such as sulfuric acid, hydrochloric acid, and nitric acid.
Discussion and Conclusions
In summary, this review observed that most EOL PVM recovery methods had been singularly focused on improving specific technical functioning of an already existing method rather than incorporating other crucial aspects such as their environmental sustainability. The team also noted that an inconsistent and insufficient volume of EOL PVMs and a lack of established recovery facilities might limit the scope of conducting large-scale studies.
The authors believe that the present-day unit economic computations for the EOL PVM recovery may not be accurate representations of future operations. Currently, the uncertainty about the profitability, the primary recovery process, and the required investments profoundly limit the economic incentives for the EOL PVM recoveries. Future work in this field should consider the integration of the proposed solutions into a larger industrial-scale system while also taking into account its overall operational feasibility, economic viability, and sustainability.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Tan, J.; Jia, S.; Ramakrishna, S. End-of-Life Photovoltaic Modules. Energies 2022, 15, 5113. https://www.mdpi.com/1996-1073/15/14/5113