Reviewed by Alex SmithJul 25 2022
The global warming caused by greenhouse gas emissions is considerable. Fluorine-containing gases, such as so-called per- or polyfluorinated hydrocarbons, or PFCs, as well as carbon dioxide (CO2), play a crucial role in this process.
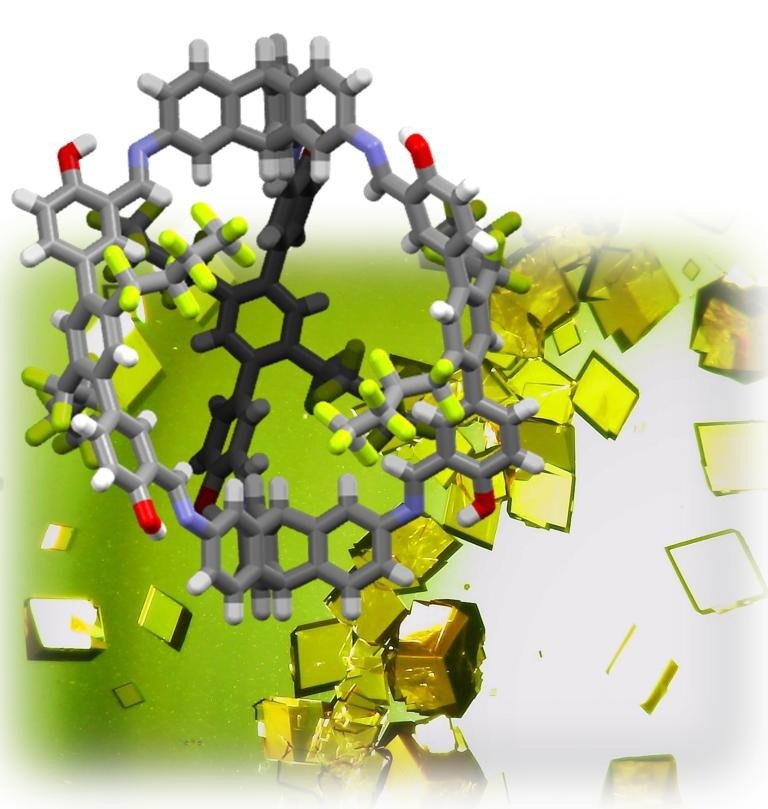 In the background: light microscopy images of the single-crystal structures of the shape-persistent organic cage compound. In the front: ball-and-stick model of the single-crystal structure, grey: carbon, white: hydrogen, red: oxygen, blue: nitrogen, green: fluorine. Image Credit: Prof. Dr. Michael Mastalerz.
In the background: light microscopy images of the single-crystal structures of the shape-persistent organic cage compound. In the front: ball-and-stick model of the single-crystal structure, grey: carbon, white: hydrogen, red: oxygen, blue: nitrogen, green: fluorine. Image Credit: Prof. Dr. Michael Mastalerz.
New crystalline materials that can specifically adsorb the molecules with such carbon–fluorine linkages have recently been produced by scientists at Heidelberg University’s Institute of Organic Chemistry under the direction of Prof. Dr. Michael Mastalerz. The Heidelberg researchers are hoping that PFCs may be specifically bound to and recovered from these porous crystals.
Polyfluorinated carbons are chemical compounds of different lengths in which fluorine atoms have partially or completely replaced the hydrogen atoms of alkanes. Chemically, these atoms are quite stable. They are not often found in nature and are mostly employed for etching procedures in the semiconductor industry, eye surgery, and in the field of medicine as contrast enhancers for certain ultrasound diagnostics.
Unlike CO2, which is integrated in natural material cycles, PFCs accumulate in the atmosphere and stay there for several thousands of years before breaking down.
Dr. Michael Mastalerz, Professor, Institute of Organic Chemistry, Heidelberg University
PFCs thus have a considerably higher potential for causing global warming than carbon dioxide does since one PFC molecule has an effect that is roughly equivalent to 5,000 to 10,000 CO2 molecules. Consequently, polyfluorinated hydrocarbons, in the researcher’s opinion, become a persistent issue that is both causing and exacerbating global warming.
Prof. Mastalerz has created a novel kind of crystalline material that can very selectively attach polyfluorinated hydrocarbons to its internal surface with the help of his research team at Heidelberg University’s Institute of Organic Chemistry. The shape-persistent organic cage structures that carry fluorine-containing side chains on the linked struts serve as the foundation for the porous crystals.
Through fluorine–fluorine interactions with the PFC molecules, these side chains react in accordance with the “like attracts like” principle and ensure that they are coated on the inner surface of the material.
The Heidelberg scientists established via their investigations that the crystals they generated bind certain fluorine-containing gases like octafluoropropane or octafluorocyclobutane 1,500–4,000 times more strongly than dinitrogen, the primary component of air. These figures reflect very high selectivities to bind such PFCs, according to Prof. Mastalerz.
Presently, Prof. Mastalerz and his group are focusing on boosting the crystals’ selectivity and adapting the procedure to different fluorinated gases, including those used in anesthesia for patients. “I see enormous potential for development in this area,” emphasizes the researcher.
Prof. Mastalerz hopes that the polyfluorinated hydrocarbons may be recovered at the point of the application using the adsorbent.
The study was financed by the German Research Foundation.
Journal Reference:
Tian, K., et al. (2022) Highly Selective Adsorption of Perfluorinated Greenhouse Gases by Porous Organic Cages. Advanced Materials. doi.org/10.1002/adma.202202290.