Writing in the journal Materials, a team of scientists from the National Institute for Cryogenics and Isotopic Technologies in Romania has investigated the use of iodine-doped graphene oxide as electrocatalysts.
Study: Iodine-Doped Graphene Oxide: Fast Single-Stage Synthesis and Application as Electrocatalyst. Image Credit: Dhujmontra/Shutterstock.com
Fuel Cell Technologies
Fuel cells are a key sustainable energy storage solution that is helping to drive current green technological development. These highly efficient devices electrochemically reduce hydrogen to produce electrical energy and have been explored for both stationary and portable/wearable technologies.
A rapidly emerging technology, fuel cells have been widely proposed for applications in multiple industries. Several fuel cell technologies have been developed in recent years, with proton exchange membrane fuel cells showing particular promise as a clean and sustainable energy solution. These fuel cell variants are highly efficient and produce nearly no pollution.
Low-temperature proton exchange membrane fuel cells have emerged in the past few years for stationary applications and light vehicles. However, the use of platinum as the catalyst of choice for electrodes in fuel cell technologies hinders their full commercialization due to its extremely prohibitive cost.
Minimizing costs is key to ensuring that fuel cell technologies become a widespread solution for the current climate crisis. To overcome the cost limitations of these technologies, research has focused on the development of alternative platinum-free electrode materials.
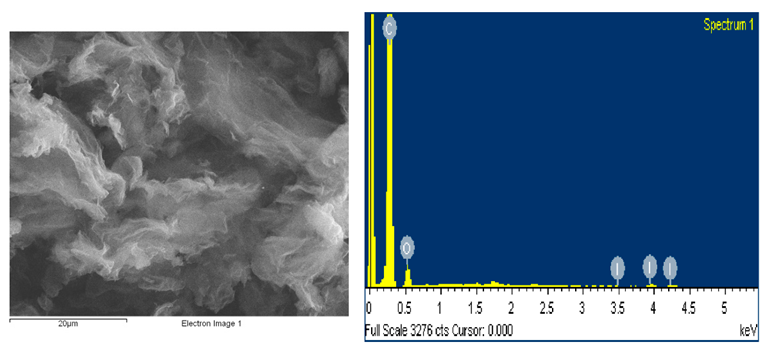
SEM microstructure and energy dispersive X-ray spectroscopy spectrum of prepared I-doped graphene oxide. Image Credit: Marinoiu, A et al., Materials
Utilizing Graphene Materials
Graphene, which is a nanostructured carbon-based material, has emerged in recent years as a viable low-cost alternative to platinum for electrodes in fuel cells. This innovative nanomaterial possesses remarkable physical, chemical, and electrochemical properties that have made it an ideal material for applications such as electrolyzers and solar cells.
Consisting of single-atom thick sheets of carbon, graphene has a hexagonal lattice structure. The oxidized form of graphene is known as graphene oxide, and this material has been identified as a suitable graphene production route.
Graphene oxide displays favorable characteristics such as a strongly hydrophilic behavior due to the presence of abundant functional groups. Furthermore, it displays good dispersion capacities due to its hydrophilicity and strong electrostatic charge. Reduced graphene oxide can be produced via several routes, including by photo-thermal, thermal, and chemical means.
Functionalizing Graphene Materials with Halogen Doping
Functionalizing graphene involves changing its carbon structures using heteroatoms such as halogens. This produces organic reduction reaction catalysts with enhanced catalytic activity, which can be used in fuel cells. Moreover, functionalization can create defects within the graphene structure which improve the electrochemical performance of reactions and thermal and mechanical stability of fuel cells in both alkaline and acidic environments.
Amongst the different halogens considered for graphene electrode functionalization, iodine can significantly enhance the electrocatalytic activity of organic reduction reactions in fuel cells. Iodine forms stable diatomic molecules which share an electron pair and reversibly dissociate at elevated temperatures. Indeed, iodinated anions are the strongest reducing agent amongst stable halogens.
However, from a synthesis point of view, doping graphene-based materials with iodine atoms is a highly challenging endeavor. Several techniques have been developed in recent years, but they are hindered due to being multi-step processes that are difficult to scale. Amongst the solutions proposed to overcome the associated challenges with iodine doping, microwave-assisted techniques have emerged in recent years as viable methods.
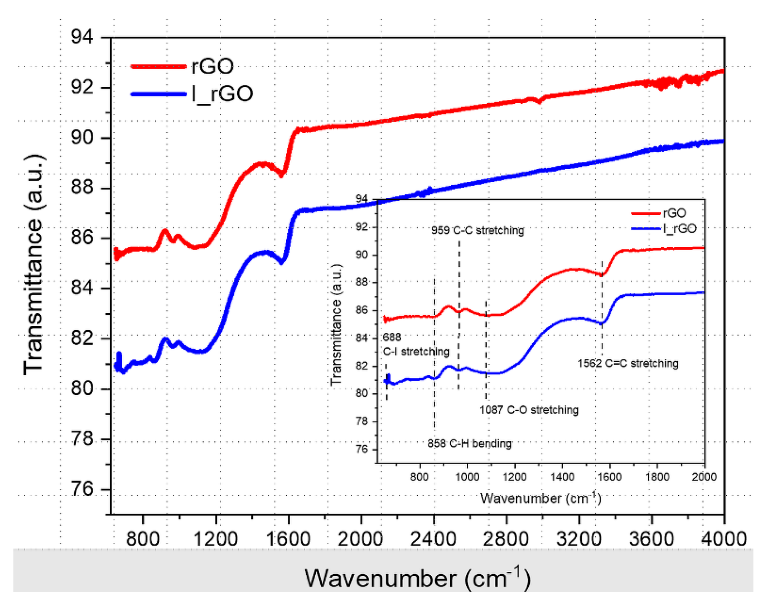
Overlaid ATR−FTIR rGO and I/rGO spectra with the fingerprint zone in the inset. Image Credit: Marinoiu, A et al., Materials
The Study
To the author’s knowledge, their paper represents the first research into applying microwave processes to iodine doping and reduction of graphene oxide to produce functionalized fuel cell organic reduction reaction electrocatalysts. A major advantage of microwave-assisted processes for this purpose is their reduced energy, time, and cost demands.
The process developed in the paper utilizes a faster, simpler, more economical, and efficient protocol under atmospheric pressure conditions. Under mild conditions, the microwave-assisted process highlighted in the research synthesizes a canvas-like iodine/reduced graphene oxide structure from graphene oxide. Thus, a low-cost, efficient alternative to platinum-based catalysts has been developed.
Study Findings
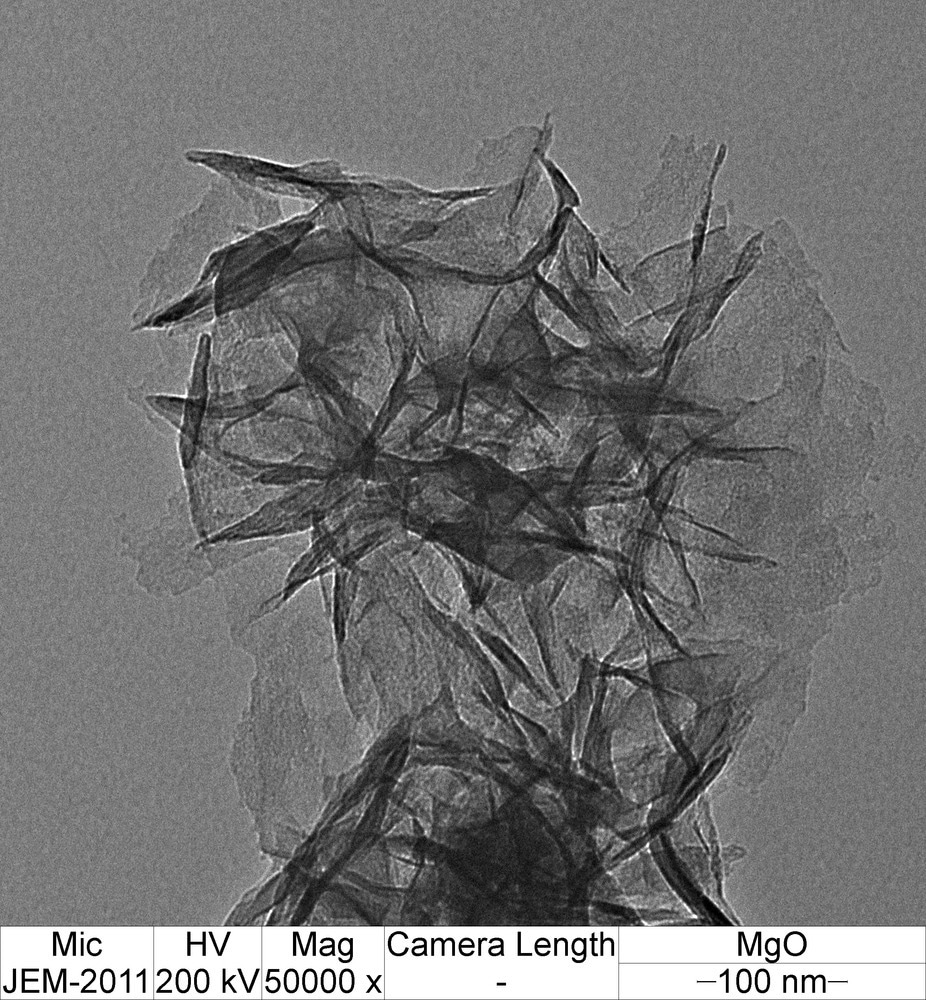
Iodine-doped graphene was obtained in the study, with specific structural and morphological properties. The microwave-assisted doping and functionalization process was achieved in a single step, reducing the complexity of preparation methods, giving it a distinct advantage over conventional synthesis methods. Temperature, pressure, power, reaction time, and doping concentration were optimized.
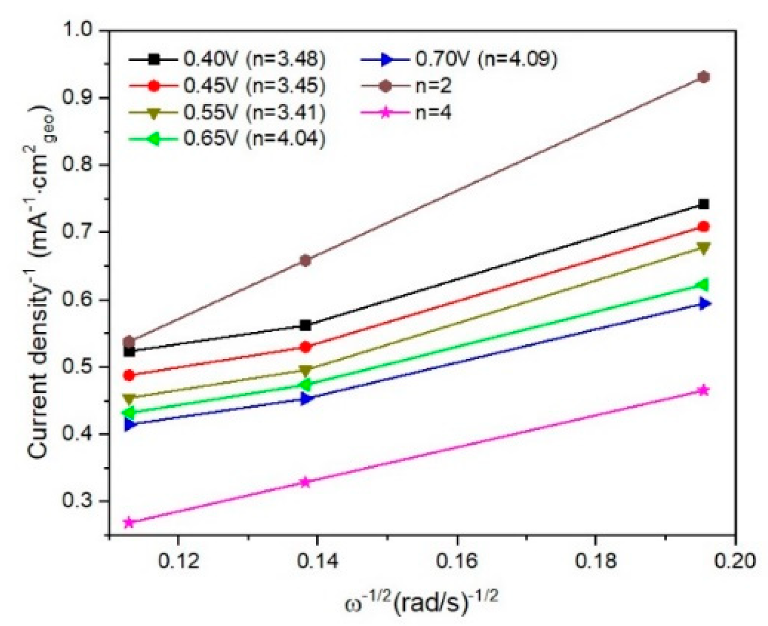
Fitted K–L plots for I/rGO determined from linear sweep voltammograms. Image Credit: Marinoiu, A et al., Materials
The organic reduction reaction electrochemical activity of the produced electrocatalytic material was confirmed using rotation disk electrode polarization and cyclic voltammetry techniques. Iodine doping, promoted by load transfer complex formation, seems to govern the enhanced organic reduction reaction activity of the prepared materials. These complexes improve the graphene’s functionalization capacity.
With an extremely short reaction time of 15 minutes and mild processing conditions, the microwave-assisted iodine-doped electrocatalytic materials presented in the paper provide a route toward low-cost and highly efficient fuel cell electrodes with enhanced organic reduction reaction activities. Green synthesis methods will improve the cost-effectiveness and environmental friendliness of fuel cell electrode manufacture and, consequently, fuel cells themselves.
Further Reading
Marinoiu, A et al. (2022) Iodine-Doped Graphene Oxide: Fast Single-Stage Synthesis and Application as Electrocatalyst Materials 15(17) 6174 [online] mdpi.com. Available at: https://www.mdpi.com/1996-1944/15/17/6174
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.