 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Sep 14 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Sep 14 2022In an article recently published in the open-access journal Materials, researchers discussed the sintering of magnesium and strontium oxide-enriched new bioactive glass at low temperatures.

Study: Low-Temperature Sintering of a New Bioactive Glass Enriched with Magnesium Oxide and Strontium Oxide. Image Credit: nevodka/Shutterstock.com
Background
The discovery of bioactivity at the end of the 1970s signaled a turning point in the study of biomaterials. Since then, orthopedics and dentistry have made extensive use of the first bioactive glass (BG), 45S5 Bioglass® (45S5), for biomedical use. Sadly, BGs have inherited several drawbacks from ordinary glasses, including brittleness and, in particular, the propensity to crystallize required to sinter powders or deposit bioactive coatings on the metal substrates.
According to reports, the crystallization reduces the glass' ionic release and inhibits their bioactivity, which limits how responsive these novel materials are to biological processes. In contrast to regular silicate glasses, 45S5 and BGs generally have a lot more modifier oxides, which cause the silicate network to be disrupted.
The 45S5 composition has been altered several times. Although the resulting glasses showed a reduced tendency to crystallize, the impact of these ions on the glass' reactivity is still not entirely understood. A new class of BGs has recently been created with a high temperature of crystallization and noticeable biological reaction. These materials' strong biological activity was explained by the presence of magnesium and strontium, whose therapeutic value has been supported by several investigations.
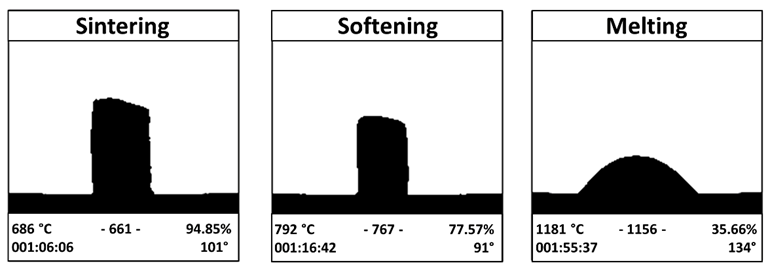
HM analysis of the BGMS_LS pressed powders. Image Credit: Bellucci, D et al., Materials
About the Study
In this study, the authors discussed the development of a novel bioactive glass (BGMS LS) that could address the major drawbacks of commercial BGs. The developed material could be sintered at extremely low temperatures without crystallizing, preserving all of its biological potential. It also contained strontium and magnesium, two elements with well-known medicinal importance.
This work enhanced the glass composition by lowering the silicon content and raising the sodium content, which resulted in an even more reactive glass known as BGMS LS. BGMS LS had one of the lowest sintering temperatures of about 686 °C because of its extraordinarily low crystallization relative to conventional BGs.
The team suggested the possibility of sintering the novel BGMS LS while leaving it entirely amorphous, which would preserve the finished product's in vivo dissolution rate, biological activity, and ionic release. This had advantages from an economic perspective. Melt-quenching was used to create BGMS LS.
To determine the material's typical temperature, the glass powders were tested using heating microscopy (HM) and differential thermal analysis (DTA). Glass particles were compressed onto disks and heated at 686 C for three hours. An in vitro test in SBF, where it was feasible to see both the significant precipitation of apatite and the creation of a silica gel (sg) layer, which preceded and followed the precipitation of the apatite itself, was used to confirm the considerable bioactivity of BGMS LS.
The researchers used the volume shrinkage D% of the glass disks after thermal treatment and the sintering parameter to evaluate the BGMS LS's sintering attitude. Through X-ray diffraction (XRD), the crystallization in sintered samples was determined. The sintered samples' hardness and Young's modulus were assessed using a micro-indentation technique and open platform apparatus.
By soaking the samples for 14 and 7 days in a simulated solution of body fluid (SBF), which had an ion concentration close to that of human plasma, the in vitro bioactivity of the BGMS LS disks was investigated. Micro-Raman spectroscopy and X-ray energy dispersion (EDS) spectroscopy were used to investigate the potential precipitation of apatite. A Raman microscope spectrometer was used to achieve this goal.
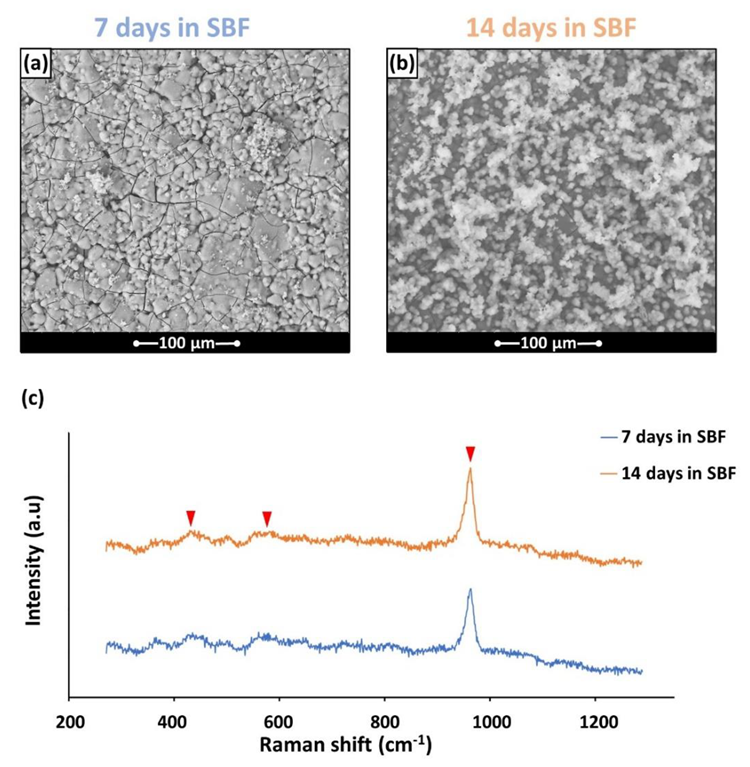
(a,b) The HA formation on BGMS_LS samples after soaking in SBF; (c) typical Raman spectra acquired on the HA precipitates. The arrows indicate the main peaks of HA. Image Credit: Bellucci, D et al., Materials
Observations
At 810 °C, BGMS LS started to devitrify, and peak crystallization occurred shortly after at an incredibly high temperature of roughly 860 °C. The processing window of BGMS LS at 200 °C was bigger than that of 45S5, and its sintering parameter Sc was positive and high, which indicated independent sintering and crystallization kinetics that happened before devitrification. The EDS results showed that except for the small fluctuations, the Ca/P ratio in the precipitate approached that of apatite, which proved the presence of apatite.
The v1 symmetric stretching vibration of phosphate anions, which was the dominant peak in the Raman spectra at 960 cm-1, dominated the spectrum. At 430 cm-1 and 590 cm-1, two additional large peaks were seen that gradually emerged from the background. The notable bioactivity of BGMS LS and its low-temperature sintering could be attributed to several competing elements that come from the glass's composition.
The glass network was weaker due to the reduced SiO2 content compared to 45S5, which favored the glass's reactivity. The greater thermal stability was a result of the reduced Na2O level. MgO's positive impact was enhanced by the glass sintering due to its stronger field than calcium. Strontium made the novel multi-component BGMS LS more viscous and increased the entropy of mixing, which encouraged the persistence of a disordered amorphous state and prevented crystallization. These factors made the noticeable decrease in alkaline oxides and the inclusion of strontium and magnesium, whose biological importance was established, an exciting solution to get around the well-known drawbacks of conventional bioactive glasses.
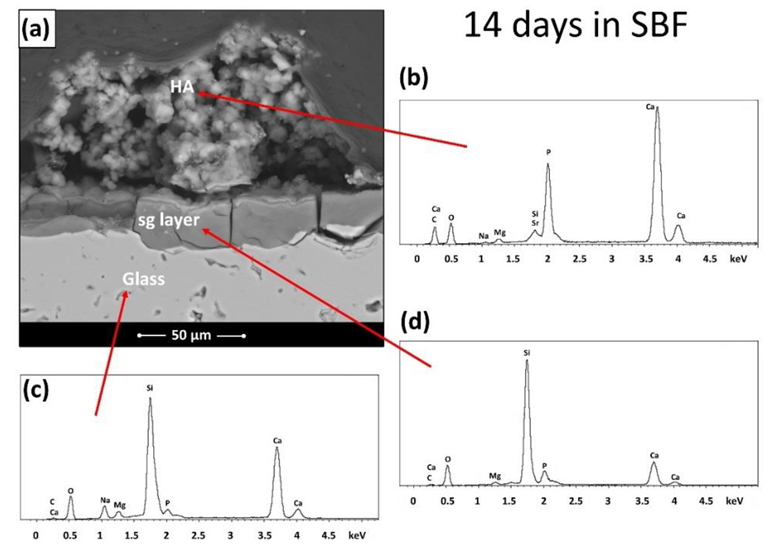
BGMS_LS samples after immersion in SBF for 14 days: (a) cross section and (b–d) EDS spectra. Image Credit: Bellucci, D et al., Materials
Conclusions
In conclusion, this study elucidated that the novel BGMS LS had outstanding thermal stability because of its composition, which enabled the glass to sinter at a temperature significantly lower than that of the standard 45S5. The sintered glass was entirely amorphous despite the heat treatment, allowing for the creation of highly bioactive scaffolds and coatings.
The authors mentioned that BGMS LS is extremely promising, especially for the creation of goods that need to undergo a heat process, like scaffolds for bone tissue regeneration. They also stated that the biological reactivity of magnesium and strontium could make the novel glass even more promising in light of a clinical application for BGMS LS.
References
Bellucci, D., Cannillo, V., Low-Temperature Sintering of a New Bioactive Glass Enriched with Magnesium Oxide and Strontium Oxide. Materials, 15(18), 6263 (2022). https://www.mdpi.com/1996-1944/15/18/6263
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.