Reviewed by Mila PereraOct 26 2022
Professors Jianping Guo and Ping Chen of the Dalian Institute of Chemical Physics (DICP) at the Chinese Academy of Sciences, along with Professor An’an Wu of Xiamen University, led a team of researchers that discovered how lithium hydride facilitated hydrogenolysis of anilines to arenes.
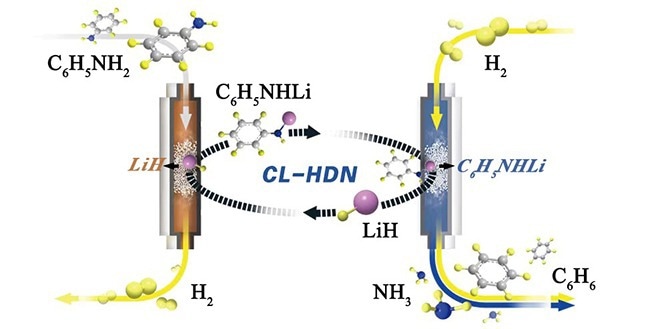 Lithium hydride mediated transition metal-free hydrogenolysis of anilines to arenes. Image Credit: Yongli Cai
Lithium hydride mediated transition metal-free hydrogenolysis of anilines to arenes. Image Credit: Yongli Cai
On September 15th, 2022, this study was published in the Journal of the American Chemical Society.
Organic and biomacromolecules frequently include C-N bonds. C-N bond activation is involved in many important chemical processes. The cleavage of C-N bonds, especially the sp2C-N bond, remains challenging due to the high C-N bond dissociation energy, intensive coordinating ability, and inferior leaving ability of the NH2 group.
For the hydrogenolysis of aniline, the researchers developed a lithium hydride (LiH)-mediated chemical looping mechanism that decoupled the overall hydrodenitrogenation (HDN) reaction into a series of distinct phases.
LiH deprotonated aniline at the initial step of the loop, resulting in the formation of lithium anilide and H2. The second stage involved producing benzene and lithium amide (LiNH2) by subjecting the lithium anilide to dihydrogen at elevated temperatures. Finally, the chemical cycle was completed by hydrogenating LiNH2 in a flow of H2 to regenerate the LiH.
Contrary to transition metals, the researchers discovered that benzene was the dominant denitrogenated product in this process. As a result, the complete hydrogenation of aromatic rings was achieved.
Therefore, they could produce highly denitrogenated products at a pace equivalent to transition metal catalysts while operating at lower temperatures and pressures.
According to the computational studies, the hydride (H–) of LiH served as a nucleophile to attack the α-sp2C atom during the cleavage of the C-N bond, and the Li cation connected with the deformed aromatic ring through a cation-π interaction.
This work provides a new strategy for C-N bond activation and may help the design and development of new materials or catalysts for the HDN as well as other important chemical transformations.
Jianping Guo, Researcher, Dalian Institute of Chemical Physics
Journal Reference
Cai, Y., et al. (2022) Transition Metal-Free Hydrogenolysis of Anilines to Arenes Mediated by Lithium Hydride. Journal of the American Chemical Society. doi:10.1021/jacs.2c05586.