The first self-healing polymer has been produced by material scientists at RIKEN using a commercially available compound. The team offers a promising method for increasing the toughness and reducing the environmental impact of different commercial polymers for a variety of applications.
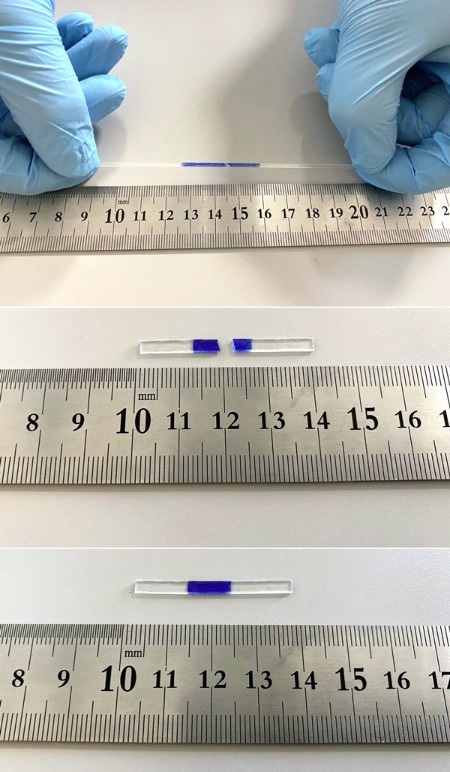 A sample of self-healing polyisoprene (bottom) was cut into two pieces (middle) and then pressed together for 15 seconds. After one minute, the sample could be stretched to more than double its original length without breaking (top). Image Credit: 2022 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
A sample of self-healing polyisoprene (bottom) was cut into two pieces (middle) and then pressed together for 15 seconds. After one minute, the sample could be stretched to more than double its original length without breaking (top). Image Credit: 2022 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Polymers that can repair themselves after damage would last longer, which would lower costs and the environmental impact. Most current methods for creating self-healing polymers involve reversible chemical reactions, but these typically involve challenging synthesis procedures.
Furthermore, in some environments, such as water and acidic and alkaline solutions, self-healing mechanisms based on chemical reactions might not function.
In a perfect world, material scientists would like to synthesize readily accessible materials into polymers that can self-heal in a variety of environments.
The most widely used synthetic polymer in the world is polyolefin, which includes polyethylene and polypropylene.
Polyolefins are all around us; they are used for food packaging, clothing, automobiles, and electronic and medical devices. Making self-healable polyolefins would enhance the lifetime, safety, and environmental impact of materials used in many applications.
Zhaomin Hou, Center for Sustainable Resource Science, RIKEN
Now, Hou and colleagues have succeeded in creating a type of polyolefin polyisoprene, a synthetic substitute for rubber latex used in tires, rubber bands, and shoe soles, that demonstrates a strong, physical self-healing mechanism.
It is important to note that their self-healing polyisoprene is created from the same isoprene building block as regular polyisoprene.
The team divided a block of their polymer in half, bringing the two halves together at room temperature for one minute, and this demonstrated the polymer’s capacity for self-healing.
Their success was due to the use of a rare-earth catalyst to combine two distinct polyisoprene microstructures. One microstructure was soft, while the other was comparatively hard. For the self-healing property, a hard microstructure to soft microstructure ratio of about 7:3 was ideal.
The research team hypothesizes that the network of hard microstructures that link together to form connecting points for molecules is what causes self-healing.
Hou added, “The ultimate goal is to use easily available commodity monomers to produce tough self-healing polymers that are capable of spontaneously repairing when they sustain mechanical damage in real-world environments without any external input. We believe that this work offers unprecedented insights to help achieve this goal.”
Journal Reference:
Wang, H., et al. (2022) Making Polyisoprene Self-Healable through Microstructure Regulation by Rare-Earth Catalysts. Angewandte Chemie International Edition. doi:10.1002/anie.202210023.