In daily human life, batteries play a major role everywhere, right from cell phones and smart watches to the growing number of electric vehicles. The majority of such devices utilize renowned lithium-ion battery technology.
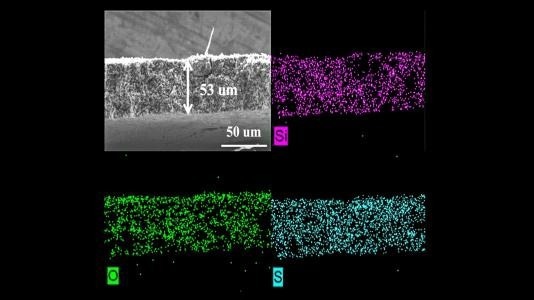
Image shows microstructure and elemental mapping (silicon, oxygen and sulfur) of porous sulfur-containing interlayer after 500 charge-discharge cycles in lithium-sulfur cell. Image Credit: by Guiliang Xu/Argonne National Laboratory.
Lithium-ion batteries have advanced significantly but have a few familiar disadvantages, including overheating, short lifetimes, and supply chain difficulties for a few raw materials.
At the US Department of Energy’s (DOE) Argonne National Laboratory, researchers are studying solutions to such problems by testing new materials in battery construction. One such material is sulfur. Sulfur is known to be more abundant and could hold more energy compared to conventional ion-based batteries.
In a new study, scientists advanced sulfur-based battery research by making a layer inside the battery that increases energy storage capacity while overcoming previous issues with sulfur batteries that led to corrosion.
A hopeful battery design couples a sulfur-containing positive electrode (cathode) along with a lithium metal negative electrode (anode). In the middle, those components present are the electrolyte or the substance enabling ions to pass between the battery’s two ends.
Early lithium-sulfur (Li-S) batteries did not execute well since sulfur species (polysulfides) dissolved into the electrolyte, resulting in its corrosion. This polysulfide shuttling effect has a negative impact on battery life and reduces the number of times the battery can be recharged.
To avoid this polysulfide shuttling, scientists in the past have tried positioning a redox-inactive interlayer between the cathode and anode. The term “redox-inactive” implies that the material does not experience reactions like those in an electrode.
However, this protective interlayer is known to be dense and heavy, thereby decreasing energy storage capacity per unit weight for the battery. Also, it does not sufficiently decrease shuttling. This has demonstrated been a barrier to the commercialization of Li-S batteries.
To address this issue, scientists engineered and tested a porous sulfur-consisting interlayer. Tests performed in the laboratory displayed initial capacity around three times higher in Li-S cells with this active, instead of inactive, interlayer. In a highly impressive manner, the cells consisting of the active interlayer retained a high capacity of more than 700 charge-discharge cycles.
Previous experiments with cells having the redox-inactive layer only suppressed the shuttling, but in doing so, they sacrificed the energy for a given cell weight because the layer added extra weight. By contrast, our redox-active layer adds to energy storage capacity and suppresses the shuttle effect.
Guiliang Xu, Study Co-Author and Chemist, Argonne National Laboratory
To additionally study the redox-active layer, the team performed experiments at the 17-BM beamline of Argonne’s Advanced Photon Source (APS), a DOE Office of Science user facility. The data collected from exposing cells with this layer to X-Ray beams enabled the team to determine the advantages of the interlayer.
The data verified that a redox-active interlayer could decrease shuttling, reduce harmful reactions that take place within the battery, and increase the capacity of the battery to hold more charge and last for more cycles.
These results demonstrate that a redox-active interlayer could have a huge impact on Li-S battery development. We’re one step closer to seeing this technology in our everyday lives.
Wenqian Xu, Beamline Scientist, Advanced Photon Source, Argonne National Laboratory
In the future, the research group wishes to assess the growth potential of the redox-active interlayer technology.
We want to try to make it much thinner, much lighter.
Guiliang Xu, Study Co-Author and Chemist, Argonne National Laboratory
The authors who contributed to the study are Khalil Amine, Tianyi Li, Xiang Liu, Guiliang Xu, Wenqian Xu, Chen Zhao, and Xiao-Bing Zuo.
This study was sponsored by the DOE’s Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office Battery Materials Research Program, and the National Research Foundation of Korea.
Journal Reference
Lee, B-J., et al. (2022) Development of high-energy non-aqueous lithium-sulfur batteries via redox-active interlayer strategy. Nature Communications. doi.org/10.1038/s41467-022-31943-8.