A promising and environmentally friendly method for addressing the energy crisis and lowering emissions from fossil fuels is the conversion of solar energy into hydrogen energy. A lead-free perovskite photocatalyst for solar energy to hydrogen conversion was recently created by a research team from the City University of Hong Kong (CityU).
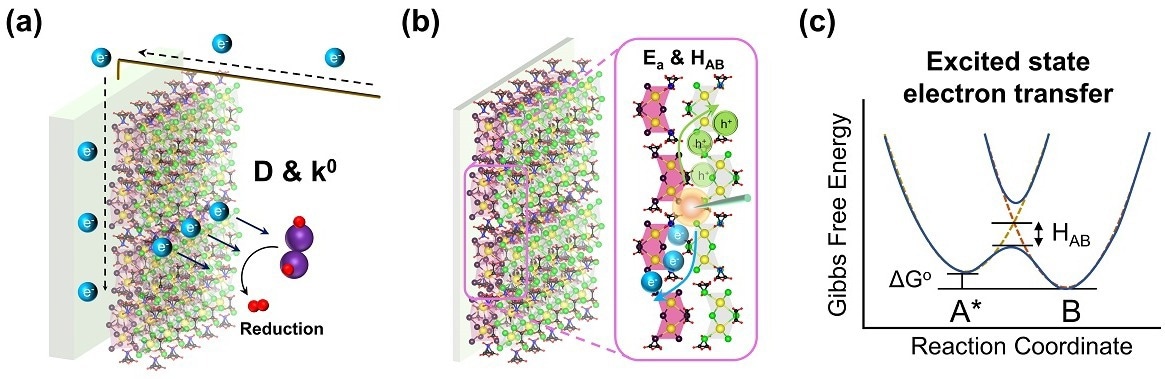
Schematic illustration of a photoelectrode deposited with a bismuth-based halide perovskite photocatalyst. (a) Heterogeneous electron-transfer kinetics during a photoelectrochemical hydrogen iodide splitting reaction. (b) Exciton transport dynamics of photogenerated electron-hole pairs; (c) Interfacial dynamics of excited state electron transfer. Image Credit: Dr. Sam Hsu’s research group/City University of Hong Kong.
Most significantly, they revealed the dynamics of the interfaces between solid-solid (between halide perovskite molecules) and solid-liquid (between a halide perovskite and an electrolyte) during the photoelectrochemical hydrogen production process.
The most recent discoveries provide a path toward creating a future hydrogen fuel production process that is more solar-powered and effective.
Due to its abundance, high energy density, and environmental friendliness, hydrogen is regarded as a better and more promising alternative to non-renewable energy sources. A promising alternative to photoelectrochemical water splitting is to split hydrohalic acid using photocatalysts powered by solar energy.
The majority of catalysts made of transition metal oxides or metal are unstable under acidic conditions, so the long-term stability of photocatalysts is a significant challenge.
Lead-based hybrid perovskites are used to overcome this stability issue, but the high solubility in water and toxicity of lead limits their potential for widespread applications. Bismuth-based perovskites, in contrast, have been confirmed to provide a non-toxic, chemically stable alternative for solar-fuel applications, but the photocatalytic efficiency needs to be enhanced.
Dr. Sam Hsu Hsien-Yi, Study Corresponding Author and Assistant Professor, School of Energy and Environment and the Department of Materials Science and Engineering, City University of Hong Kong
Dr. Hsu and his colleagues recently created a bismuth-based halide perovskite with a bandgap funneling structure for highly efficient charge-carrier transport, driven by the desire to design an effective and stable photocatalyst.
It is a mixed-halide perovskite where the iodide ion distribution gradually decreases from the surface to the interior, forming a bandgap funnel structure that facilitates a photo-induced charge transfer from the interior to the surface for a successful photocatalytic redox reaction.
With a platinum co-catalyst under visible light irradiation, this newly created perovskite exhibits a hydrogen generation rate enhanced up to approximately 341±61.7 mol h–1. The results were released six months ago.
“We wanted to explore the dynamic interactions between the halide perovskite molecules and those at the interface between the photoelectrode and the electrolyte, which remained unknown. Since photoelectrochemical hydrogen production involves a catalytic process, highly effective hydrogen generation can be achieved by intense light absorption using a semiconductor as a photocatalyst with a suitable energy band structure and efficient charge separation, facilitated by an external electrical field formed near the semiconductor-liquid interface,” said Dr. Hsu.
The team used temperature-dependent time-resolved photoluminescence to examine the energy transfer of electron-hole pairs between the perovskite molecules to learn about the dynamics of exciton transfer.
To demonstrate the efficiency of electron transport through the solid-liquid interfaces between a perovskite-based photoelectrode and the electrolyte, they also measured the diffusion coefficient and electron transfer rate constant of halide perovskite materials in the solution.
Dr. Hsu stated, “We demonstrated how our newly designed photocatalyst can effectively achieve high-performance photoelectrochemical hydrogen generation as a result of efficient charge transfer.”
The team’s experiment also demonstrated that charge separation and transfer between the electrode and electrolyte interfaces were more effectively accomplished by bandgap funneling structured halide perovskites.
Faster photoelectrochemical activity on the photoelectrode’s surface is made possible by the improved charge separation, which can cause charge carriers to migrate onto the surface of halide perovskites deposited on the conductive glasses as the photoelectrode.
As a result, under light irradiation, the efficient charge transfer inside the bandgap funnel-structured halide perovskites displayed increased photocurrent density.
“Uncovering the interfacial dynamics of these novel materials during the process of photoelectrochemical hydrogen generation is a crucial breakthrough. An in-depth understanding of the interfacial interactions between halide perovskites and liquid electrolytes can build a scientific foundation for researchers in this field to further investigate the development of alternative and useful materials for solar-induced hydrogen production,” Dr. Hsu further stated.
The findings were reported in the academic journal Advanced Materials. The research study was chosen to be highlighted on the inside back cover of Advanced Materials.
Dr. Hsu is the study’s corresponding author. Dr. Tang Yunqi, a recent graduate of Dr. Sam Hsu’s lab, and Mr. Mak Chun-hong, a Ph.D. candidate under Dr. Hsu’s supervision, are the co-first authors. Professor Kai Ji-jung, Dr Zhao Shijun, and Mr Zhang Jun, all from the City University of Hong Kong’s Department of Mechanical Engineering, are additional collaborators.
The Innovation and Technology Commission, the Shenzhen Science Technology and Innovation Commission, the City University of Hong Kong, and the Research Grants Council of Hong Kong provided financial support for the study.
Journal References
Tang, Y., et al. (2022) Unravelling the Interfacial Dynamics of Bandgap Funneling in Bismuth-Based Halide Perovskites. Advanced Materials. doi:10.1002/adma.202207835
Tang, Y., et al. (2022) Bandgap Funneling in Bismuth-Based Hybrid Perovskite Photocatalyst with Efficient Visible-Light-Driven Hydrogen Evolution. Small Methods. doi:10.1002/smtd.202200326.