Researchers from the University of Cambridge and University College London (UCL) have discovered a new type of ice that resembles liquid water the most out of all the forms, and which may hold the key to understanding this most well-known of liquids.
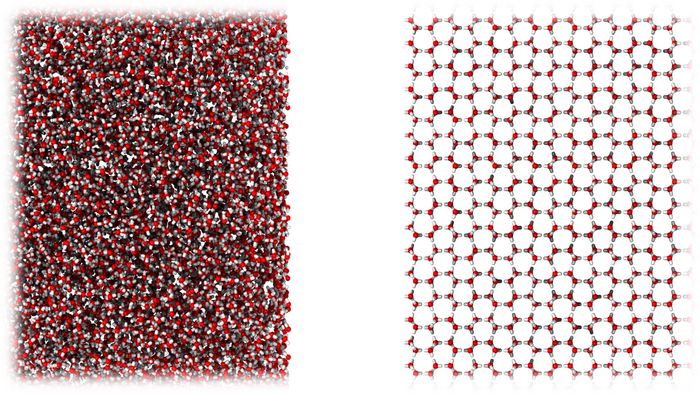
A new form of ice very similar in molecular structure to liquid water (left), compared to ordinary crystalline ice (right). Image Credit: University of Cambridge
Ice in its new form is amorphous. Amorphous ice has molecules that are disorganized and have a liquid-like appearance, as opposed to conventional crystalline ice, which has molecules that arrange themselves in a regular manner.
The team described in the journal Science how they developed a new type of amorphous ice using experimentation and computer modeling to produce an atomic-scale model. The studies employed a method known as ball-milling, which entails putting metal balls in a steel container to crush crystalline ice into microscopic particles.
Although it is frequently used to create amorphous materials, ice has never been subjected to ball-milling.
The researchers discovered that ball-milling produced an amorphous type of ice with a density comparable to liquid water and a condition that resembled water in solid form, unlike all other known ice. Their new ice was given the term medium-density amorphous ice (MDA).
The researchers used computer simulation to comprehend the mechanism at the molecular level. The team successfully developed a computational model of MDA by repeatedly randomized shearing crystalline ice to mimic the ball-milling process.
Our discovery of MDA raises many questions on the very nature of liquid water and so understanding MDA’s precise atomic structure is very important. We found remarkable similarities between MDA and liquid water.
Dr Michael Davies, Study Co-Author, University of Cambridge
A Happy Medium
It has been proposed that amorphous ices serve as models for liquid water. Amorphous ice has historically been divided into two primary categories: high-density amorphous ice and low-density amorphous ice.
The names imply that there is a significant density difference between them. The concept of liquid water has been based on this density gap in addition to the fact that liquid water has a density that is in the center. It has contributed to the idea that water is made up of two different liquids, one with a high density and the other with a low density.
The names imply that there is a significant density difference between them. The concept of liquid water has been based on this density gap in addition to the fact that liquid water has a density that is in the center. It has contributed to the idea that water is made up of two different liquids, one with a high density and the other with a low density.
Christoph Salzmann, Study Senior Author and Professor, Physical and Materials Chemistry, University College London
A High-Energy Geophysical Material
Where might MDA possibly be found in nature, given its discovery? In this investigation, it was revealed that shear stresses are essential for producing MDA. The team hypothesizes that due to the tidal forces generated by gas giants like Jupiter, ordinary ice could experience similar shear forces in the icy moons.
Additionally, MDA exhibits a remarkable quality unique from other types of ice. They discovered via calorimetry that MDA releases a remarkable amount of heat as it recrystallizes to common ice.
The heat produced by MDA’s recrystallization could contribute to the tectonic movements. This finding demonstrates that water could be a high-energy geophysical material more generally.
Amorphous ice in general is said to be the most abundant form of water in the universe. The race is now on to understand how much of it is MDA and how geophysically active MDA is.
Angelos Michaelides, Study Lead Author and Professor, Yusuf Hamied Department of Chemistry, University of Cambridge
Journal Reference:
Rosu-Finsen, A., et al. (2023) Medium-density amorphous ice. Science. doi:10.1126/science.abq2105.