Hydrogen spillover is the dynamic movement of surface-adsorbed hydrogen species from hydrogen-abundant areas to hydrogen-weak areas. It has a vital role to play in numerous hydrogen-involved reaction processes.
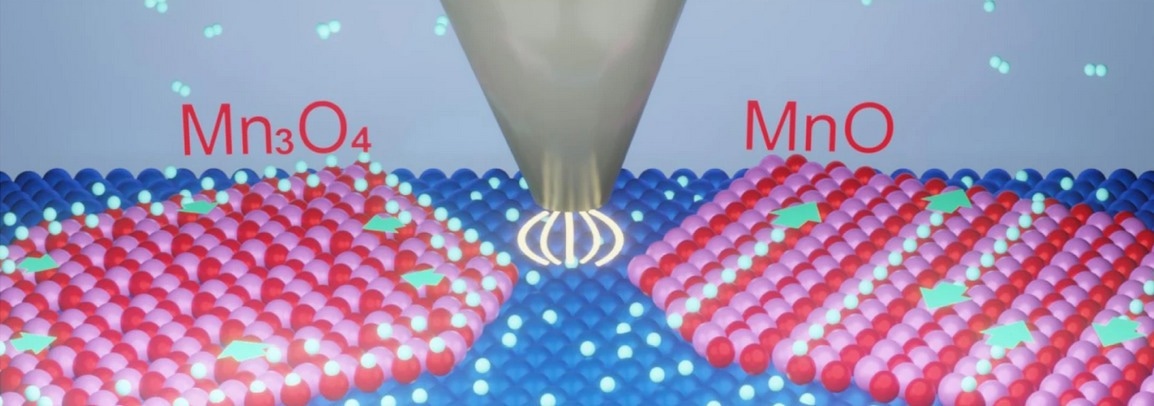
Schematic of high-pressure scanning tunneling microscopy (HP-STM) using an STM tip to probe the hydrogen spillover on MnO and Mn3O4 surfaces in the H2 atmosphere. Image Credit: Rentao Mu and Yijing Liu.
To improve the catalytic performance of hydrogen-involving reactions, it is crucial to comprehend the full mechanism of hydrogen spillover and expose how hydrogen moves and what aspects regulate hydrogen conductivity on solid surfaces.
Recently, a team of researchers headed by Prof. Rentao Mu and Prof. Qiang Fu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) directly witnessed the speeding up of hydrogen spillover through the surface-lattice-confinement effect. This study was published in the journal Nature Communications on February 4th, 2023.
The scientists built stripe-like MnO(001) and grid-like Mn3O4(001) monolayers on a Pt(111) substrate and examined their hydrogen spillover. They learned that hydrogen species from Pt spread unidirectionally along the stripes on MnO(001), whereas it displayed an isotropic pathway on Mn3O4(001).
Additionally, by employing dynamic surface imaging in the H2 atmosphere, they exposed that hydrogen spread four times more quickly on MnO than in the case on Mn3O4, which was stimulated by the one-dimension surface-lattice-confinement effect.
Theoretical calculations showed that an even and medium O-O distance was ideal for hydrogen diffusion while low-coordinate surface O atom blocked it.
Our study illustrates the surface-lattice-confinement effect of oxide catalysts on hydrogen spillover and provides a promising route to improve the hydrogen spillover efficiency.
Qiang Fu, Professor, Dalian Institute of Chemical Physics, Chinese Academy of Sciences
Journal Reference
Liu, Y., et al. (2023) Direct observation of accelerating hydrogen spillover via surface-lattice-confinement effect. Nature Communications. doi.org/10.1038/s41467-023-36044-8.