Compared to ordinary lithium-ion batteries, solid-state lithium-sulfur batteries have the potential for substantially better energy densities and increased safety. However, solid-state battery performance is currently poor, with slow charging and discharging being one of the key problems.
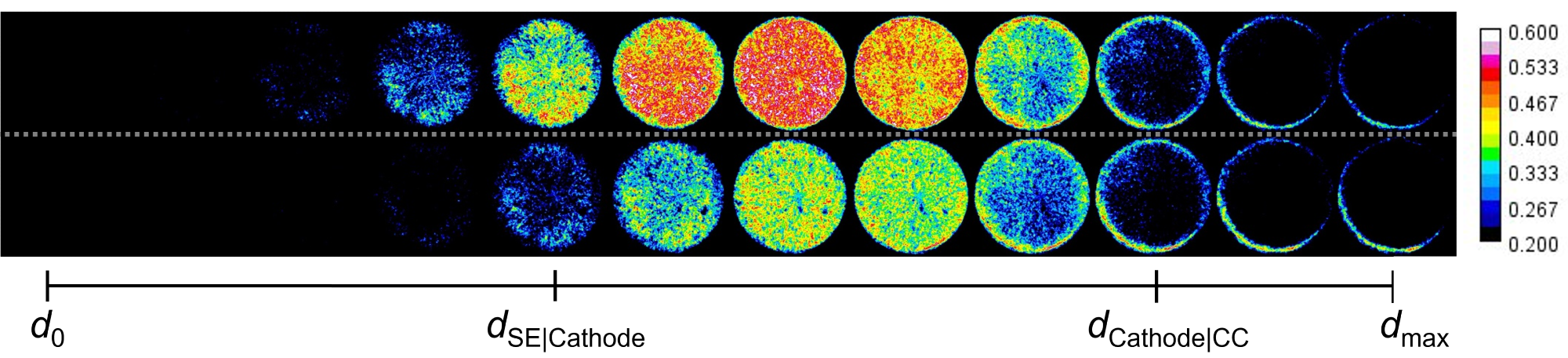 The change in neutron attenuation in the cathode, shows where lithium accumulates: at the top during discharging and at the bottom during charging. d0 is the boundary to the solid electrolyte and dmax is the boundary between the cathode and the current collector. Image Credit: © HZB
The change in neutron attenuation in the cathode, shows where lithium accumulates: at the top during discharging and at the bottom during charging. d0 is the boundary to the solid electrolyte and dmax is the boundary between the cathode and the current collector. Image Credit: © HZB
A new HZB study reveals that slow lithium ion transport within a composite cathode is to blame for the delayed charging and discharging.
The researchers created a unique cell to study the movement of lithium ions between the anode and cathode of a solid-state lithium-sulfur battery. Because lithium is difficult to identify using X-Ray methods, HZB physicists Dr. Robert Bradbury and Dr. Ingo Manke used neutrons, which are particularly sensitive to lithium, to investigate the sample cell.
Researchers used neutron radiography and neutron tomography procedures on the CONRAD2 instrument at the Berlin neutron source BER II in collaboration with Dr. Nikolay Kardjilov, HZB. The study also included groups from Giessen (JLU), Braunschweig (TUBS), and Jülich (FZJ).
Lithium Ions Observed Directly
We now have much better idea what is limiting the battery performance. We see from the operando neutron radiography data that there is a reaction front of lithium ions propagating through the composite cathode confirming the negative influence of a low effective ionic conductivity.
Dr. Robert Bradbury, Physicist, Helmholtz-Zentrum Berlin für Materialien und Energie
Furthermore, images from 3D neutron tomography indicate trapped lithium localized near the current collector during recharging.
“This results in a diminished capacity because only some of the lithium is transported back when the battery is charged,” adds Dr. Robert Bradbury
The observed lithium distribution corresponded well with a model based on porous electrode theory.
“What we observe here in the neutron imaging data correlates well with the relevant electronic and ionic conductivity conditions from the model,” adds Bradbury.
Bottleneck Identified
These findings reveal a previously unknown development barrier for solid-state batteries, demonstrating that cathode composites have constraints due to delayed ionic transport. The goal now is to improve ion delivery within the cathode composite.
Without direct visualization of the reaction front inside the cathode composite this effect might have gone unnoticed, despite its importance for solid-state battery development.
Dr. Robert Bradbury, Physicist, Helmholtz-Zentrum Berlin für Materialien und Energie
Journal Reference:
Bradbury, R., et al. (2023). Visualizing Reaction Fronts and Transport Limitations in Solid‐State Li–S Batteries via Operando Neutron Imaging. Advanced Energy Materials. doi.org/10.1002/aenm.202203426.