Alloying is a common and powerful methodology to increase the catalytic activity of Co catalysts toward alcohol oxidation (AHO) via electronic effects. In addition, vacancies also demonstrate superior AHO functioning due to their strong adsorption capacity to alcohols.
 Using an arc-melting method, researchers have synthesized CoSi alloy with rich-vacancy for the the base- and solvent-free selective oxidation of various alcohols, which exhibited excellent catalytic acitvity and stability. Image Credit: Chinese Journal of Catalysis
Using an arc-melting method, researchers have synthesized CoSi alloy with rich-vacancy for the the base- and solvent-free selective oxidation of various alcohols, which exhibited excellent catalytic acitvity and stability. Image Credit: Chinese Journal of Catalysis
Thus, a mixture of the alloying and vacancies would be a potential approach to further fabricate outstanding catalysts for AHO. Nevertheless, the controllable synthesis of alloy with rich vacancies is still difficult.
Now, a research group headed by Prof. Kai Yan from Sun Yat-Sen University controllably fabricated a CoSi alloy with rich-vacancies (AM-CoSi) via a solvent-free arc-melting (AM) method in less than 5 minutes for solvent- and base-free selective oxidation of alcohols. The findings were reported in Chinese Journal of Catalysis.
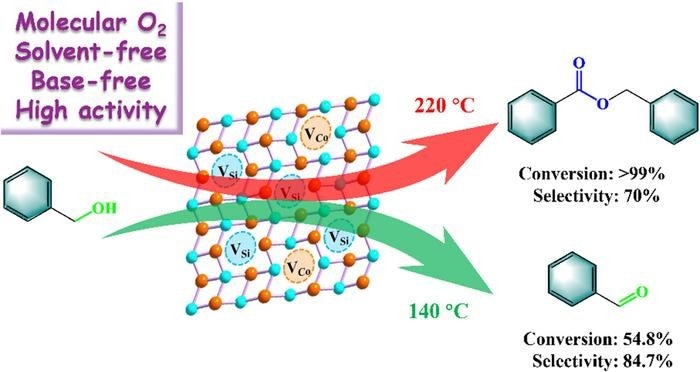
At first, electron paramagnetic resonance (EPR), X-Ray absorption fine structure (XAFS), and aberration-corrected high-angle annular dark field scanning transmission electron microscope (AC HAADF-STEM) verified the efficient synthesis of AM-CoSi alloy using both Co vacancy (Cov) and Si vacancy (Siv), where Siv is in the majority. The creation of vacancies in AM-CoSi is perhaps due to the quick cooling at the time of the preparation process. In addition, the Co element in AM-CoSi is in the metallic state form.
The as-prepared AM-CoSi alloy catalysts were used for Benzyl alcohol (BAL) solvent- and base-free oxidation. By controlling the catalytic reaction temperature, benzyl benzoate (BBE) and benzaldehyde (BZH) can be selectively regulated as the main production. At 54.8% conversion of BAL, 84.7% selectivity to BZH can be obtained at 140 °C within 5 bar O2 for 6 hours. With a maximum of 99.9% conversion of BAL, 70% selectivity to BBE can be attained at 220 °C within 9 bar O2 for 24 hours, the maximum value reported until now.
Six aromatic alcohols with electro-withdrawing and electro-donating groups were also solvent- and base-free oxidated to produce aldehydes with the use of AM-CoSi alloy with outstanding universality and high catalytic performance. In addition, CoSi alloy retained high stability in the oxidation process.
Furthermore, the creation route of BBE was analyzed and BBE is achieved via the direct esterification of BAL with benzoic acid (BAD) using AM-CoSi at high temperatures. BAD can be attained at high temperatures instead of low temperatures (<140 °C) via more oxidation of BZH from BAL conversion.
The factors for AM-CoSi catalytic capacity were also used. Compared with their monometallic Co, counterpart Si, and standard CoSi alloy (S-CoSi) for solvent- and base-free oxidation, the CoSi alloyed structure enhances the BAL oxidation. AM-CoSi displays a greater performance for the production of BBE due to the significant roles of vacancy in AM-CoSi. Furthermore, density functional theory (DFT) calculations show that Si vacancies and the alloyed structure of AM-CoSi add up to the creation of BBE.
Journal Reference:
Zhao, Z., et al. (2023). Facile synthesis of CoSi alloy catalysts with rich vacancies for base- and solvent-free aerobic oxidation of aromatic alcohols. Chinese Journal of Catalysis. https://doi.org/10.1016/S1872-2067(23)64418-3