Investigators have succeeded in producing slow electrons in a solution. In the future, such electrons might aid in the efficacy of certain chemical reactions.
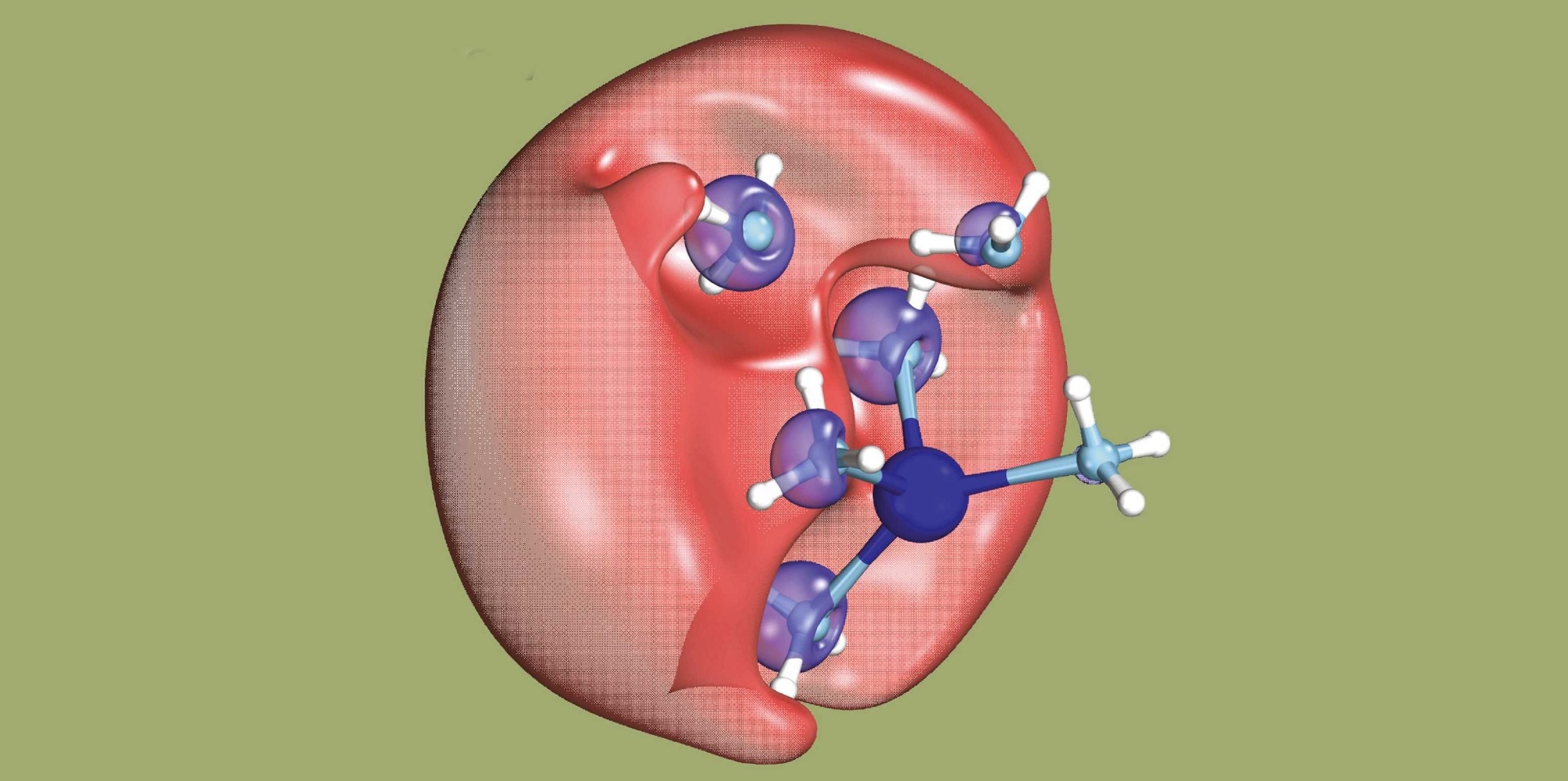
Here, two electrons are briefly united as a dielectron (red) surrounded by solvent molecules. The dielectron cannot be localized more precisely. One of the electrons will subsequently leave this area. Image Credit: Hartweg S et al. Science 2023
The international team of scientists basically set out to identify a mysterious chemical object: a dielectron in solution. A dielectron comprises two electrons; however, unlike an atom, it lacks a nucleus. Until now, researchers have been unable to detect such an object directly.
While researching with dielectrons, the scientists, headed by ETH Zurich Professor Ruth Signorell, unintentionally found a novel way to create slow electrons. These can be used to start specific chemical reactions.
Dielectrons are highly unstable. In less than one trillionth of a second, they split into two electrons. As the researchers demonstrated, one of these electrons remains in place while the other—which has low energy and hence moves slowly—moves away. The new method is unique in that it enables the investigators to regulate the kinetic energy of the electron and, thereby, its speed.
Dielectrons Occupy Cavities
The investigators dissolved sodium in (liquid) ammonia and subjected this solution to UV light to create the dielectrons. This exposure causes an electron from an ammonia molecule to combine with an electron from a sodium atom, resulting in the formation of a dielectron.
The dielectron temporarily settles in a small cavity in the solution. The researchers were able to demonstrate that when the dielectron breaks up, one of the electrons moves at a speed defined by the wavelength of the UV light employed.
Some of the UV light energy has been transferred to the electron.
Ruth Signorell, Professor, ETH Zurich
The ETH Zurich scientists collaborated with colleagues from the University of Freiburg in Germany, the SOLEIL synchrotron in France, and Auburn University in the United States on this research.
Examining Reactions and Radiation Damage
Low-kinetic-energy electrons are intriguing for a variety of reasons. One effect of slow electrons is that they cause radiation damage to human tissue. Mobile electrons can form in this tissue as a result of X-Rays or radioactivity, for example.
They can then bind to DNA molecules, causing chemical reactions. Producing such slow electrons in the lab will allow researchers to better investigate the mechanisms that cause radiation damage.
However, the human body is not the only place chemical reactions are stimulated by a compound accepting a free electron. One example is the manufacturing of synthetic cortisone and other steroids.
Making it possible to employ UV light as a very simple method of directly producing slow electrons in a solution, as well as manipulating the energy of the electron, would make it easier to accurately assess these reactions in the future. Chemists may even be able to optimize processes by employing UV light to increase the kinetic energy of electrons.
Journal Reference
Hartweg, S., et al. (2023). Solvated dielectrons from optical excitation: An effective source of low-energy electrons. Science. doi.org/10.1126/science.adh0184.