At present, the manufacturing of chemicals contributes 40% of all energy used in production. Also, the method leads to toxic solvent waste that pollutes the environment and causes health risks to animals and humans.
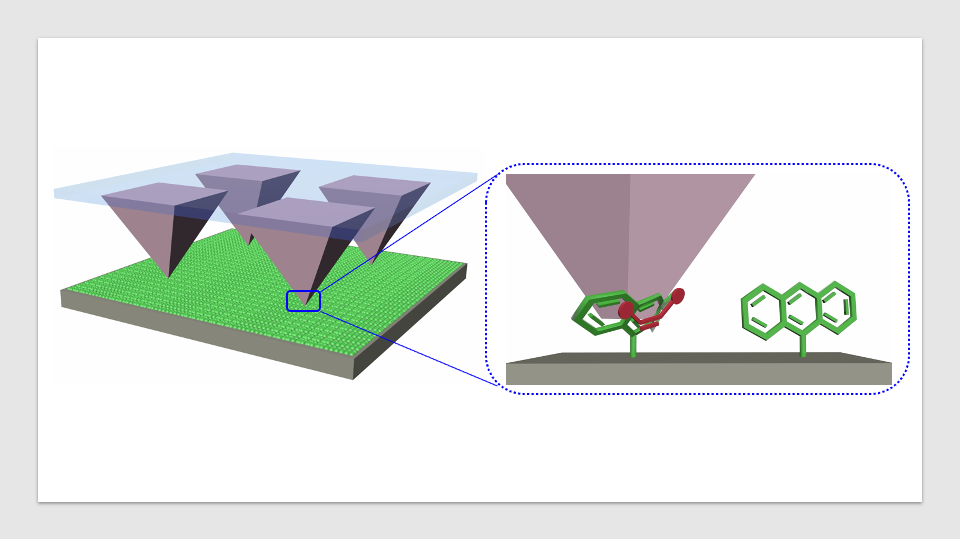 Tip arrays transfer a dienophile molecules (red) onto an anthracene-modified (green) surface. Upon contact, the tips form nanoreactors, where pressure is applied that accelerates the Diels-Alder cycloaddition reactions. For their study, the authors took monolayers of molecules placed on silicon wafers and pushed reactive molecules into them using tip arrays, which created new chemicals. The experimental setup allowed the researchers to precisely control the pressure between the molecules, which led to a new understanding of what occurs in these reactions. Image Credit: Yerzhan Zholdassov
Tip arrays transfer a dienophile molecules (red) onto an anthracene-modified (green) surface. Upon contact, the tips form nanoreactors, where pressure is applied that accelerates the Diels-Alder cycloaddition reactions. For their study, the authors took monolayers of molecules placed on silicon wafers and pushed reactive molecules into them using tip arrays, which created new chemicals. The experimental setup allowed the researchers to precisely control the pressure between the molecules, which led to a new understanding of what occurs in these reactions. Image Credit: Yerzhan Zholdassov
A recently published study in the journal Science discusses a novel mechanochemistry approach that has the capacity to manufacture chemicals without such harmful effects.
Scientists with the Nanoscience Initiative at the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC), the University of Pennsylvania, and the University of California-Merced applied a unique method that hopes to boost the use of mechanochemistry in large-scale manufacturing. The technique utilizes nanotechnology and organic chemistry to push molecules together and produce chemicals without using expensive solvents that contaminate the environment.
The results of the study team have major consequences for several production sectors, along with the production of pharmaceuticals and materials for a range of industrial and medical purposes.
This is a really exciting breakthrough, because the discovery makes mechanochemistry a reliable means of producing chemicals, and it allows us to do so without the harmful byproducts and large energy demands of current manufacturing techniques.
Adam Braunschweig, Study Lead Author and Professor, Department of Chemistry, ASRC Nanoscience Initiative and Hunter College, City University of New York
When we pushed on the molecules, we found that they twisted into new, more reactive shapes that require less energy to combine and produce a desired chemical.
Yerzhan Zholdassov, Study First Author and Doctoral Student, Braunschweig Lab, City University of New York
The experiment let scientists quantify the force required to generate a predictable and dependable chemical reaction and prove that mechanochemistry is a scalable and viable technique for producing chemicals more sustainably and economically. Also, the new technique can be applied to produce new drugs and materials that cannot be produced with the use of the existing techniques that depend on solvents.
This discovery was not possible without chemists teaming up with mechanical engineers in a truly cross-disciplinary way. The chemists were critical to designing and conducting the experiments, but we had to combine their forefront chemistry knowledge with advanced mechanics analysis to understand—through experiments and theory—how mechanical forces are accelerating chemical reactions here. The teamwork made the difference.
Robert Carpick, Study Co-Author and Professor, Department of Mechanical Engineering and Applied Mechanics, University of Pennsylvania
This study was financially supported by the National Science Foundation (NSF) Center for the Mechanical Control of Chemistry along with the NSF Division for Innovation in Biological Research.
Journal Reference:
Zholdassov, Y. S., et al. (2023) Acceleration of Diels-Alder reactions by mechanical distortion. Science. https://doi.org/10.1126/science.adf5273