New research headed by Prof. Ji Qian and Prof. Renjie Chen (Department of Energy and Environmental Materials, School of Materials Science and Engineering, Beijing Institute of Technology) demonstrates how when fluorinated cyclic carbonate (DFEC) is led into an ether electrolyte as an SEI-forming additive, the altered electrolyte can enhance the interface of the Li metal anode. This may help to attain long cycling stability and high efficiency of LMBs.
 Schematics illustrating the role of DFEC in improving solid electrolyte interphase (SEI) and affection on Li deposition in ether electrolyte. Image Credit: Science China Press
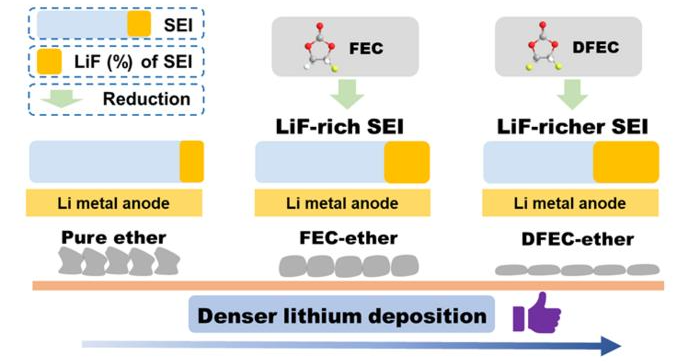
Schematics illustrating the role of DFEC in improving solid electrolyte interphase (SEI) and affection on Li deposition in ether electrolyte. Image Credit: Science China Press
LMBs are considered the most potential next-generation battery system, thanks to the low electrode potential (-3.04 V vs. SHE) and high specific capacity (3860 mAh g−1) of the Li metal anode. There are, however, many restrictive aspects that hinder the development of LMBs, such as Li dendrite growth and serious volume effect of Li anode, side reaction between Li anode and electrolyte, etc., resulting in low Coulombic efficiency (CE) and poor cycle life.
Stable solid electrolyte interphase (SEI) is vital in achieving long cycling stability and high efficiency of LMBs. Fine-tuning SEI via electrolyte optimization is considered a cheap and successful approach to enhancing Li metal anode interface. Hence, it is important to develop an electrolyte formulation that can make a stable SEI; the key is the choice of film-forming additives and solvents.
Recently, Prof. Renjie Chen and Prof. Ji Qian suggested an ether-ester mixed electrolyte in which trans-difluoroethylene carbonate (DFEC) was introduced into the ether electrolyte as a film-forming additive. First of all, ether electrolyte has good anti-reduction stability with Li metal. In addition, owing to the lower LUMO level of DFEC, it can be favorably minimized at the time of the initial cycle, making LiF-rich SEI on the Li metal anode.
LiF-rich SEI can constrain the growth of lithium dendrite, relieve side reactions, and induce dense lithium deposition. Due to the aforementioned advantages, the LMBs utilizing modified electrolyte exhibit stable cycling and high-efficiency performance. The study’s first author is Tianyang Xue, a graduate student at Beijing Institute of Technology, and the corresponding authors are Prof. Renjie Chen, Prof. Ji Qian, and Prof. Xingming Guo.
Thus, a few inferences arise for developing an electrolyte to enhance the high efficiency and long cycling stability of LMBs. This study discovers the interphase chemistry of LMBs and offers essential understanding for more studies on the novel electrolyte system for LMBs.
Journal Reference:
Xue, T., et al. (2023). Tailoring fluorine-rich solid electrolyte interphase to boost high efficiency and long cycling stability of lithium metal batteries. Science China Chemistry. https://doi.org/10.1007/s11426-022-1623-2.