High-entropy alloys (HEAs), which were first developed in 2004, are alloys made up of several primary elements in almost equiatomic proportions. They have exceptional features, including high strength, ductility, and excellent wear-and-tear resistance even at high temperatures because of their particular chemical makeup, which results in a high level of chemical disorder, or entropy.
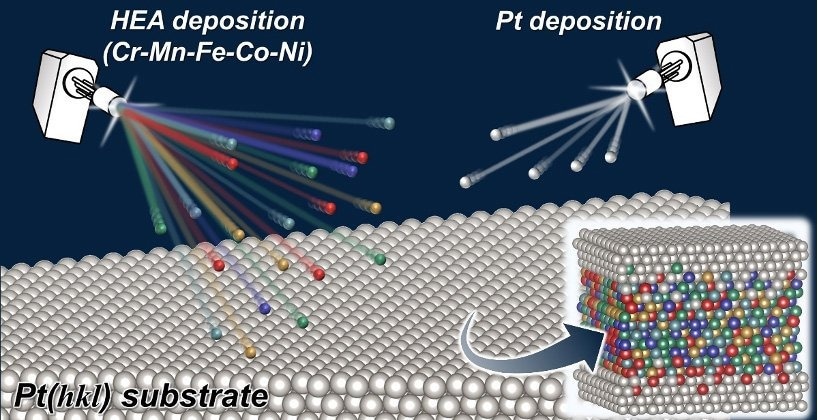 A schematic Illustration of Structural-controlled Pt-high-entropy-alloy (Pt-HEA) Model Catalyst Synthesis. Image Credit: Yoshihiro Chida et al.
A schematic Illustration of Structural-controlled Pt-high-entropy-alloy (Pt-HEA) Model Catalyst Synthesis. Image Credit: Yoshihiro Chida et al.
Researchers have devoted a lot of time and effort to creating unique HEAs to enhance the performance of electrocatalyst materials.
HEAs can have intricate atomic-level surface designs due to their composition of several constituent elements. The surface characteristics of materials frequently determine their catalytic activity; hence it is important to understand this intricacy.
For this reason, scientists are trying to figure out how HEAs’ catalytic capabilities relate to their atomic configuration.
The atomic-level structure of HEA surfaces can now be controlled, and their catalytic capabilities can be tested, thanks to the development of a novel experimental platform by a multidisciplinary research team. On July 26th, 2023, a report on their discovery was published in the journal Nature Communications.
In our study we made thin layers of an alloy called a Cantor alloy, which contains a mix of elements (Cr-Mn-Fe-Co-Ni), on platinum (Pt) substrates. This produced a model surface for studying a specific reaction called the oxygen reduction reaction (ORR).
Toshimasa Wadayama, Study Co-Author and Professor, Graduate School of Environmental Studies, Tohoku University
The researchers investigated the surface atomic structure of the Pt-HEAs and investigated the ORR characteristics using sophisticated imaging techniques. They found that the Pt-HEA surfaces outperformed surfaces composed of a platinum-cobalt alloy in ORR.
This suggests that the remarkable catalytic characteristics of Pt-HEAs are due in part to the atomic arrangement and distribution of elements on the surface, which results in a “pseudo-core-shell-like structure.”
Wadayama and his team emphasize the broad application of their findings, both to other nanomaterials and any constituent elements.
Wadayama added, “Our newly constructed experimental study platform provides us with a powerful tool to elucidate the detailed relationship between multi-component alloy surface microstructures and their catalytic properties. It is valid for clarifying the precise correlations among the atomic-level, surface microstructure and electrocatalytic properties of HEAs of any constituent elements and ratios and, thus, would provide reliable training datasets for materials informatics. The platform is applicable not only to electrocatalysis but also in various fields of functional nanomaterials.”
By utilizing Pt-HEA nanoparticles that aim to improve electrochemical surface areas, the group expects to convert this platform into usable electrocatalysis in the future.
Journal Reference:
Chida, Y., et al. (2023) Experimental study platform for electrocatalysis of atomic-level controlled high-entropy alloy surfaces. Nature Communications. doi:10.1038/s41467-023-40246-5