Carbon capture and utilization (CCU) technologies are critical when it comes to addressing climate change-related concerns while ensuring economic feasibility. MES has surfaced as a promising approach for reducing CO2 into biofuels and platform chemicals, holding significant potential for sustainable energy and chemical production. However, the industrial adoption of MES has been hampered by low-value products like acetate or methane and high electric power demand.
Graphical abstract. Image Credit: Environmental Science and Ecotechnology
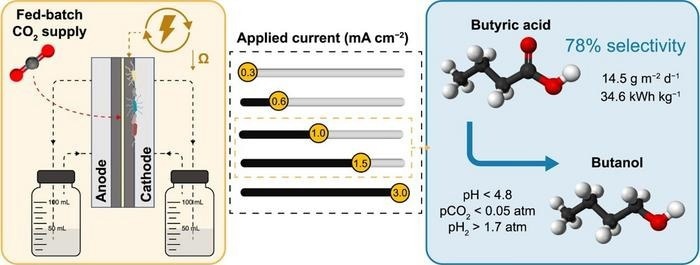
Recently published in the journal Environmental Science and Ecotechnology, scientists from the University of Girona undertook a study that focused on electrically efficient MES cells with low ohmic resistance (15.7 mΩ m2). Via a fed-batch mode, alternating the availability of high CO2 and hydrogen (H2), the team was able to efficiently promote the creation of ethanol and acetic acid.
Chain elongation lead to the selective (78% on a carbon basis) generation of butyric acid, a key chemical employed in farming, pharmaceuticals, perfumes, and the chemical industry. The study attained a remarkable average production rate of 14.5 g m−2 d−1 of butyric acid at an applied current of 1.0 or 1.5 mA cm−2.
Megasphaera sp. was itentified as the key player in the chain elongation process.Inoculating a second cell with the enriched community replicated the butyric acid production rate but with an 82% reduction in the lag phase.
Butyric acid was efficiently upgraded to butanol, which is a valued biofuel compatible with the current gasoline infrastructure and employed as a precursor in chemical and pharmaceutical industries for methacrylate and acrylate production.
Solventogenic butanol production was triggered at a pH below 4.8 by interrupting the CO2 supply and maintaining specific pH and hydrogen partial pressure conditions. With average cell voltages of 2.6–2.8 V and an electric energy need of 34.6 kWhel kg−1 of butyric acid created, the MES cell design proved highly successful.
In spite of certain restrictions owing to H2 and O2 crossover via the membrane, the study found ideal operating settings for energy-efficient butyric acid creation from CO2.
Highlights
- In fed-batch mode, low-resistance bioelectro-cells functioned galvanostatically.
- Alternating high pCO2 and pH2 promoted bioelectro-CO2 conversion to butyric acid.
- By consuming 35 kWh kg−1, a rate of 14.4 g m−2 d−1 and 78% selectivity were achieved.
- Megasphaera sp. carried out chain elongation at the cathode.
- Keeping pH2 > 1.7 atm and pH < 4.8 promoted butanol production in absence of CO2.
To summarize, this study highlights the capacity of bioelectrochemical conversion of CO2 to butyric acid and its consequent upgrade to butanol in microbial electrolysis cells.
The process holds great promise for the sustainable and economically viable production of valuable chemicals from CO2. However, further research and development are still needed to optimize the process for large-scale applications.
Nonetheless, this technology still has the potential to revolutionize chemical production while mitigating the impact of climate change.
Journal Reference:
Romans-Casas, M., et al. (2023) Selective butyric acid production from CO2 and its upgrade to butanol in microbial electrosynthesis cells. Environmental Science and Ecotechnology. doi.org/10.1016/j.ese.2023.100303