Fusion reactors, fast breeder reactors, and solar thermal power plants are being developed as low-impact, resource-free power plants. Scientists are researching the usage of components that employ liquid metal (which has good heat transfer performance) as a coolant in these power plants since they run at high temperatures with considerable heat transfer.
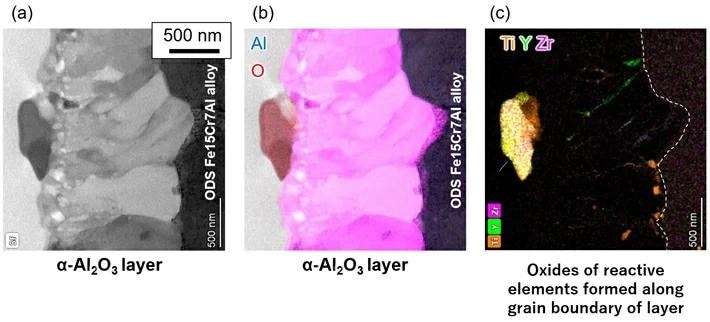 Cross-sectional microstructure of α-Al2O3 layer formed by ODS Fe15Cr7Al alloy. (a) Scanning transmission electron microscope image; (b) Energy dispersive X-Ray (EDX) elemental mapping image of aluminum and oxygen; (c) Elemental mapping image of titanium, yttrium, and zirconium by EDX analysis. Image Credit: Tokyo Institute of Technology
Cross-sectional microstructure of α-Al2O3 layer formed by ODS Fe15Cr7Al alloy. (a) Scanning transmission electron microscope image; (b) Energy dispersive X-Ray (EDX) elemental mapping image of aluminum and oxygen; (c) Elemental mapping image of titanium, yttrium, and zirconium by EDX analysis. Image Credit: Tokyo Institute of Technology
The liquid metal blanket (a metal wall inserted in the core) and the liquid metal divertor (which receives heat and discharges exhaust) are two essential components of fusion reactors that have sparked interest in revolutionary energy conversion technologies. However, finding structural materials that are chemically compatible with high-temperature liquid metals has been difficult.
Associate Professor Masatoshi Kondo of the Tokyo Institute of Technology investigated the chemical corrosion resistance of liquid metal coolants with leading structural materials. He discovered that corrosion is caused by the leaching of metallic components from materials in contact with liquid metal, in addition to the alloying of liquid metal and steel materials.
In that regard, he discovered that developing a compact protective oxide layer on the surface of structural elements of liquid metal components could significantly prevent corrosion. The creation of a persistent protective oxide layer that resists such corrosion is critical to the realization of liquid metal-based components.
The joint research team, under the direction of Associate Professor Kondo, in cooperation with Yokohama National University and the National Institute for Fusion Science, concentrated on the fact that oxide dispersion strengthened (ODS) FeCrAl alloys form an α-Al2O3 (alpha-alumina) layer with a compact structure and found elements that can encourage layer growth and also the mechanism that prevents the layer from peeling off the substrate.
In settings where liquid metal is present at high temperatures, the α-Al2O3 layer offers exceptional protection. The ODS Fe15Cr7Al alloy is an outstanding prospective structural material for next-generation power plants due to its remarkable high-temperature strength. To create an α-Al2O3 layer, the alloy can be oxidized at 1,000 °C in air for 10 hours.
It is just 1.28 micrometers thick, or roughly 1/80th the thickness of a human hair, yet it has an incredibly compact structure with an evenly distributed aluminum and oxygen content. The researchers also discovered that oxides of reactive elements, including Ti, Y, and Zr were forming in the α-Al2O3 layer simultaneously.
This is because oxides have formed in the layer as a result of the reactive components that the ODS Fe15Cr7Al alloy has scattered as microscopic oxide particles in its microstructure.
FeCrAl alloys of various types do not create these oxides in the layer, and their layer growth is sluggish. This is demonstrated by comparing the microstructure and growth rate of the oxide layer formed by the various types of FeCrAl alloys.
The “oxygen-only diffusion path” that these elongated oxides of reactive elements serve encourages the formation of new layers and strengthens barrier characteristics.
Exfoliation resistance is required for the protective layer. In this study, the researchers conducted a scratch test on the α-Al2O3 layer produced on the ODS-FeCrAl alloy to determine the amount of force necessary to scratch and remove the layer using a sharp needle. According to the findings, the ODS-FeCrAl alloy has outstanding adhesion qualities.
Figure 2 summarizes the method by which the α-Al2O3 layer becomes resistant to exfoliation. First, the oxides of reactive elements produced from the substrate toward the layer firmly grip the microstructure of the layer, similar to tent pegs, and contribute to enhanced adhesion strength. This is known as the pegging effect.
Between the α-Al2O3 layer and the substrate, an unstable interface with a jugged structure form, and the depth of this jugged interface deepens as the layer thickens. Furthermore, the deeper the jagged interface, the larger the shear force required to peel the α-Al2O3 layer, implying that the layer adhesion strength is stronger.
Layer development is fostered in a relatively non-uniform way in the pattern with the oxygen diffusion channel indicated above, leading to a deeper jagged interface and a strong anchoring effect.
Other techniques of producing oxide and other layers through a solution exist, but the layers generated in this study have greater adhesion and can survive the flow of liquid metals due to their compact structure.
Developing a small, peel-resistant barrier technology offers a bright future for increasing the service life of liquid metal components such as liquid blankets and divertors. The use of liquid metal technology in modern power plants such as fusion reactors, as well as desalination and clean-up technologies, is projected to accelerate the development of a carbon-neutral society.
Journal Reference:
Kitamura,Y., et al. (2023) Excellent adhesion of protective α-Al2O3 layer formed on ODS FeCrAl alloys. Surface and Coatings Technology. doi:10.1016/j.surfcoat.2023.129787