A research group headed by Professor Minrui Gao from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences has come up with a nickel (Ni)-based anion-exchange membrane fuel cell (AEMFC) anode catalyst that showcases high resistance to ammonia (NH3) toxicity.
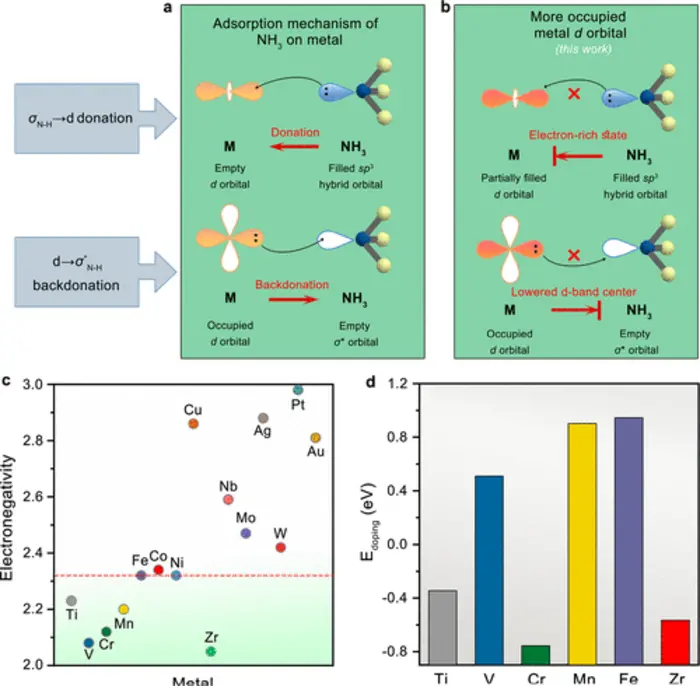 Mechanisms of NH3 poisoning and electron states regulation. Image Credit: Yehua Wang et al.
Mechanisms of NH3 poisoning and electron states regulation. Image Credit: Yehua Wang et al.
The study was published in the Journal of the American Chemical Society.
In the energy industry, hydrogen fuel cells play a significant role as a non-polluting power source along with high specific energy.
However, commercial platinum on carbon (Pt/C) catalysts are vulnerable to ammonia poisoning in hydrogen fuel cells, thereby resulting in the degradation of performance.
Much worse, the hydrogen oxidation over platinum-based catalysts consists of slow kinetics in alkaline membrane fuel cells, which synergizes with ammonia poisoning to expedite the performance degradation. Thus, a new anode catalyst along with high resistance and high activity to ammonia poisoning is an immediate need in the application of AEMFC.
Scientists reasoned that adding electrons around Ni sites can repulse lone-pair electron donation from NH3 and that integrating metal elements with smaller electronegativity compared to that of Ni can offer electrons to achieve electron-rich states.
By doping chromium (Cr) into the effective hydrogen oxidation catalyst molybdenum-nickel alloy (MoNi4), the team not only achieved the electron-rich states of Ni to repress the electron supply of σN-H→dmetal but also shifted the d-band center down to block the reverse electron supply of d→σ*N-H, both of which greatly undermines the adsorption of ammonia.
Rotating-disk electrode (RDE) tests displayed that the Cr-doped catalyst Cr-MoNi4 encountered 10,000 cycles in the existence of 2 ppm NH3 without considerable activity decay, while conventional Pt/C catalysts experienced severe decay under such conditions.
In real alkaline membrane fuel cells developed with Cr-MoNi4 as anode could retain 95% of the initial peak power density under 10 ppm NH3, in opposition to 65% of Pt/C catalyst.
It was revealed that the Cr modifier made an electron-rich state that efficiently prevents σN-H→d supply, but also downshifted the d-band center, and less d-band filling also restricting d-electron supply to the ammonia σ*N-H orbitals, thus synergistically weakening NH3 binding.
In conclusion, Cr-MoNi4 could be utilized as an efficient and highly NH3-resistant, and profitable negative HOR catalyst for AEMFC anode.
The rotating-disk electrode test disclosed that Cr-MoNi4 displayed no evident composition and structural alterations following 10,000 cycles with 2 ppm of NH3, while the platinum-carbon catalyst showed a severe loss of performance.
If put in an AEMFC, a device constructed with Cr-MoNi4 could retain 95% of its initial peak power density in the presence of 10 ppm NH3.
Attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) measurements disclosed that the Cr-free MoNi4 and the commercial Pt/C catalysts exhibited ammonia adsorption behavior at various potentials, while the Cr-modulated catalysts displayed no NH3 adsorption peaks.
Furthermore, electron energy loss spectroscopy (EELS) and electron paramagnetic resonance (EPR) measurements denoted that incorporating Cr raised the occupancy of d-band states.
Professor GAO’s research group has been working on the development and application of non-precious metal electrocatalysts for AEMFC. Such outcomes will drive future research on platinum group metal (PGM) -free catalysts that withstand poisoning by impurity gases for hydrogen fuel cells.
Journal Reference:
Wang, Y-H., et al. (2023) Efficient NH3-Tolerant Nickel-Based Hydrogen Oxidation Catalyst for Anion Exchange Membrane Fuel Cells. Journal of American Chemical Society. doi.org/10.1021/jacs.3c06903.