Chemical engineers at UC Riverside have created a fuel that only ignites when electric current is applied. It is a “safe” liquid fuel since it has no reaction to flames and cannot cause unintentional fires while being stored or transported.
 Reprinted with permission from the American Chemical Society. Image Credit: University of California, Riverside
Reprinted with permission from the American Chemical Society. Image Credit: University of California, Riverside
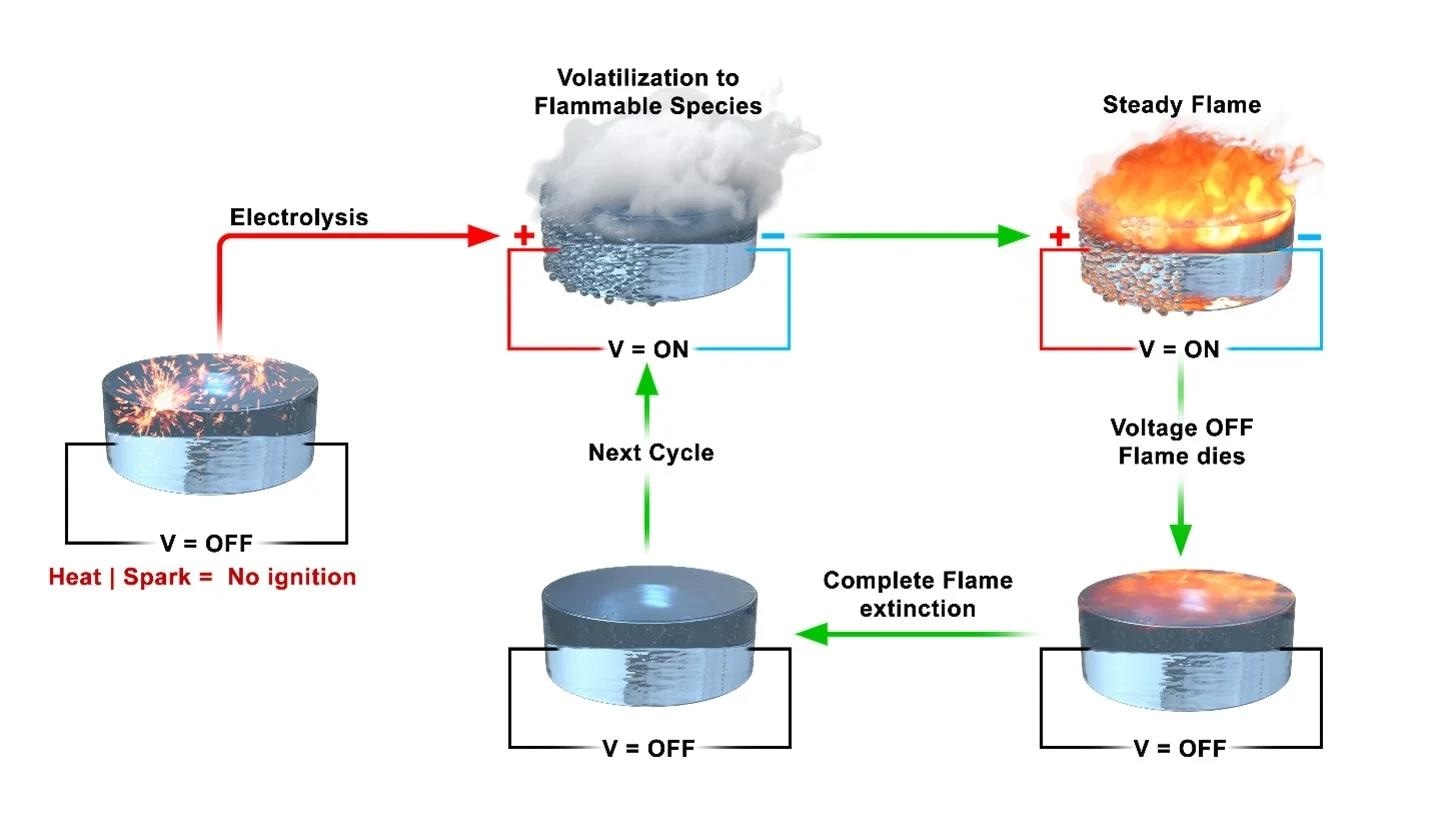
The fuel we are normally using is not very safe. It evaporates and could ignite, and it’s difficult to stop that. It is much easier to control the flammability of our fuel and stop it from burning when we remove voltage.
Yujie Wang, Study Co-Author and Doctoral Student, University of California, Riverside
The team’s process for producing the fuel is described in the Journal of the American Chemical Society publication, and further technical information is also included in the patent application.
When fuel burns, it is not the liquid that burns. Instead, when the volatile fuel molecules above the liquid come into contact with oxygen and flame, they ignite. Removing an oxygen supply will extinguish the flame, but doing it outside an engine is challenging.
If you throw a match into a pool of gasoline on the ground, it is the vapor of the gas that is burning. You can smell that vapor and you instantly know it is volatile. If you can control the vapor, you can control whether the fuel burns.
Prithwish Biswas, Study First Author and Doctoral Student, University of California, Riverside
The new fuel is based on an ionic liquid, which is a type of liquified salt.
Wang added, “It is similar to the salt we use to flavor food, which is sodium chloride. The one we used for this project has a lower melting point than table salt, low vapor pressure, and is organic.”
Once in the lab, the researchers changed the formula of the ionic liquid, substituting chlorine with perchlorate. They then lit the resultant liquid with a cigarette lighter to test if it burned.
“The temperature from a normal lighter is high enough, and if it was going to burn, it would have,” Wang noted.
The researchers next attempted a voltage application followed by a lighter flame, which did ignite.
“Once we shut off the current, the flame was gone, and we were able to repeat that process over and over again—applying voltage, seeing smoke, lighting the smoke so it burned, then turning it off. We were excited to find a system we could start and stop very quickly,” Wang added.
Increasing the voltage in the liquid produced greater flames with more energy output. As a result, the technique might function similarly to a metering or throttling system in an engine.
You can measure the combustion in this way, and cutting the voltage works like a dead man switch—a safety feature that automatically shuts down a machine if the operator becomes incapacitated.
Michael Zachariah, Study Corresponding Author and Distinguished Professor, Chemical Engineering, University of California, Riverside
The ionic liquid fuel could potentially be applied to any type of vehicle. Before it can be marketed, there are still several issues that need to be resolved. It would be necessary to test the fuel in various engine types to establish how efficient it is.
The ionic liquid has an unusual quality in that it can be combined with conventional fuel and still function as it would on its own.
Zachariah added, “But there needs to be additional research to understand what percentage can be mixed and still have it be not flammable.”
The team is pleased to have developed a fuel that is safe against unintentional, accidental fires, even if there are a lot of areas that require more investigation.
“This would definitely be more expensive than the way they currently manufacture fuels. These compounds are not normally produced in bulk, but if they were, the cost would go down. How competitive would it be? I don’t know. But if safety is important, that’s a major aspect of this. You make something safe, then there is a benefit that goes beyond the bottom line,” Zachariah concluded.
Journal Reference:
Biswas, P., et al. (2023) Electrochemical Modulation of the Flammability of Ionic Liquid Fuels. Journal of the American Chemical Society. doi:10.1021/jacs.3c04820