A liquid fuel for cars that is transformed into hydrogen by a solid catalyst is being studied by researchers at Lund University in Sweden. The used liquid is then discharged from the tank and charged with hydrogen, after which it can be utilized once more in a circular system with no emissions of greenhouse gases.
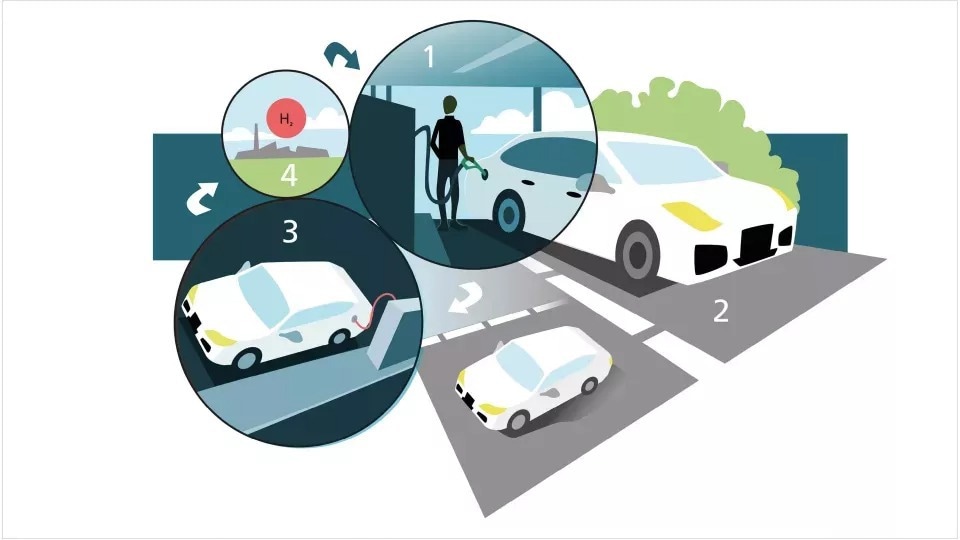 A car is refueled with a liquid containing hydrogen. The fuel passes through the catalytic converter, where hydrogen is released into a fuel cell. When the hydrogen runs out, it is drained and filled with new liquid at the gas station. Image Credit: Lund University
A car is refueled with a liquid containing hydrogen. The fuel passes through the catalytic converter, where hydrogen is released into a fuel cell. When the hydrogen runs out, it is drained and filled with new liquid at the gas station. Image Credit: Lund University
The approach has been successfully tested by Lund researchers in two studies, and although though it is still in the early stages of development, it has the potential to develop into an effective energy storage system in the future.
Our catalyst is one of the most efficient around, at least if you look at publicly available research.
Ola Wendt, Study Author and Professor, Department of Chemistry, Lund University
To lessen the effect on the environment, finding new methods of generating, storing, and changing energy is essential. This will help to cut down on carbon dioxide emissions from fossil fuels. One method makes use of the much-discussed hydrogen gas, which many believe will one day be a practical means of storing energy. Chemical bonds in nature are where energy is stored, and hydrogen has the highest energy density compared to its weight.
Wendt added, “However, gas can be difficult to handle, so we are looking at liquid fuel charged with hydrogen that can be delivered at a pump, in a way broadly similar to what happens at petrol stations today.”
The idea, often referred to as LOHC (liquid organic hydrogen carriers), is not brand-new. Finding a catalyst that is as effective as feasible and can draw hydrogen from a liquid is difficult.
A liquid that has been “charged” with hydrogen is what the system is supposed to use to function. The hydrogen is extracted from the liquid by pumping it through a solid catalyst. While the “spent” liquid is transferred to another tank, this can be utilized in a fuel cell, which transforms chemical fuel into energy. Water is the sole emission.
The used liquid can then be drained at a gas station before being refilled with fresh, charged liquid. This would likely entail mass manufacture of the material similar to what is done now in oil refineries.
“We converted more than 99 per cent of the hydrogen gas that was present in the liquid,” Wendt noted.
Additionally, scientists have been figuring out if it might be feasible to utilize the fuel for larger vehicles like buses, trucks, and airplanes.
Wendt stated, “With the large tanks that they have, it might be possible to cover almost the same distance as you can with a tank of diesel. You would also convert around 50 per cent more energy compared to compressed hydrogen.”
Isopropanol, frequently found in screenwash, and 4-methylpiperidine are the liquids utilized.
Is there a chance that this is not entirely true? Yes, there are still a lot of difficulties, at least right now. One is that the catalyst has a somewhat short life period. Another is that the catalyst is made of iridium, a valuable metal.
Wendt further stated, “But we estimate that you need about two grams of iridium per car. This could be compared to today’s exhaust-cleaning catalytic converters, which contain about three grams of platinum, palladium and rhodium, which are also precious metals.”
This is a technical answer based on fundamental investigation. Ola Wendt thinks the idea might be ready in 10 years if it were decided to pursue a finished product, assuming that it is commercially feasible and that society is interested in it.
Another issue is that most hydrogen generation today is not climate-friendly. Then, the hydrogen must be effectively stored and delivered, which is not an easy task at the moment. The use of compressed hydrogen for refueling has additional dangers. With this approach, the Lund researchers aim to find a solution.
Wendt added, “Ninety-eight per cent of all hydrogen today is fossil-based, produced from natural gas. The biproduct is carbon dioxide. From an environmental point of view, the notion of producing hydrogen for steel, batteries and fuel is pointless if it is done using natural gas.”
Wendt noted that several studies are being conducted to determine how “green hydrogen” can be created by using renewable energy to split water into hydrogen and oxygen.
Ola Wendt contends that for renewable energy and climate-friendly alternatives to succeed, political action is necessary.
Wendt concluded, “It needs to be cheaper, and it takes political decisions. Renewables have no chance of competing with something that you just dig out of the ground, where transport is almost the only cost, as is the case with fossil fuels.”
Journal Reference:
Chakrabarti, K., et al. (2023) Acceptorless dehydrogenation of 4-methylpiperidine by supported pincer-ligated iridium catalysts in continuous flow. Nature Nanotechnology. doi:10.1039/D3CY00881A
Polukeev, A. V., et al. (2022) Iridium-Catalyzed Dehydrogenation in a Continuous Flow Reactor for Practical On-Board Hydrogen Generation from Liquid Organic Hydrogen Carriers. Nature Nanotechnology. doi:10.1002/cssc.202200085