To achieve future carbon neutrality, it is crucial to generate hydrogen from clean sources, like splitting water molecules using electricity through electrolysis. Unfortunately, existing methods are inefficient, which limits the practicality of hydrogen-based technologies. A new electrocatalyst with improved electrochemical activity, reaction surface area, and durability aims to enhance the efficiency of hydrogen gas production via electrolysis.
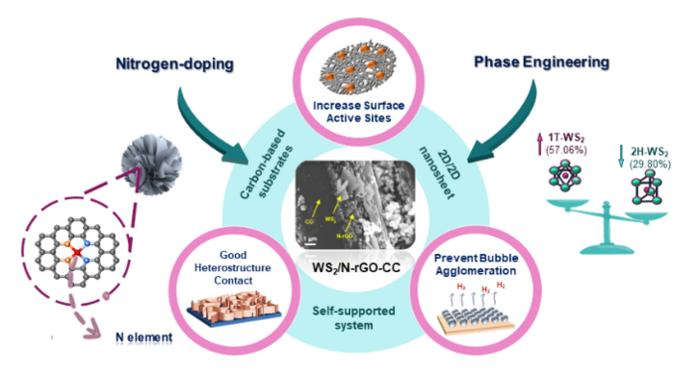
The efficiency of the WS2/N-rGO-CC electrocatalyst was optimized through the integration of nitrogen into rGO to improve contact of water with the substrate, the formation of WS2 nanoflowers to increase the surface area of the electrode and number of active sites, the incorporation of 50% DMF during the final hydrothermal synthesis step to increase the amount of metallic, phase 1T-WS2 present in the electrocatalyst and the integration of WS2 directly in the conductive materials of the electrode without the use of binders. Image Credit: Nano Research, Tsinghua University Press
Scientists from the Center of Excellence for NaNo Energy & Catalysis Technology (CONNECT) at Xiamen University in Malaysia have developed and analyzed a highly efficient and long-lasting water electrocatalyst. This electrocatalyst is constructed using tungsten disulfide (WS2), a two-dimensional material with semiconducting properties, which plays a crucial role as an electron acceptor or donor in the electrolysis reaction.
The electrocatalyst, known as WS2/N-rGO/CC, is fabricated on a carbon cloth (CC) coated with reduced graphene oxide (rGO), a two-dimensional lattice semiconductor. A small quantity of nitrogen (N) is introduced to modify the properties of the reduced graphene oxide semiconductor.
Through a hydrothermal reaction, the two-dimensional WS2 material is transformed into intricate three-dimensional structures resembling nanoflowers. These nanoflowers significantly enhance the surface area of the electrocatalyst, thereby improving the efficiency of the reaction.
The research findings were published in the journal Nano Research on September 27, 2023.
Synthesizing a self-supported electrode for the hydrogen evolution reaction in water hydrolysis is crucial because it addresses a fundamental challenge in clean energy production. Traditional methods often rely on expensive catalysts and supports, which can limit the efficiency and scalability of hydrogen production.
Feng Ming Yap, Study Lead Author and Graduate Student, School of Energy and Chemical Engineering, Xiamen University
Feng Ming Yap adds, “Our work represents a significant advancement by creating a self-supported electrode that not only enhances the electrocatalytic activity but also offers a cost-effective and sustainable solution for hydrogen generation.”
WS2/N-rGO/CC is regarded as a self-supported electrode because the active species, tungsten disulfide, is directly integrated into the conductive materials of the electrode. This electrocatalyst is synthesized without the use of polymer binders or additives, which might otherwise cover or diminish the catalyst's active sites or reduce electron conductance. This design choice maximizes the efficiency of the reaction.
The research team conducted experiments by incorporating different concentrations of dimethylformamide (DMF) in the final hydrothermal synthesis reaction to identify the optimal concentration for achieving the desired metallic 1T phase transition of WS2 in the electrode.
Notably, the electrode developed using a 50% concentration of DMF in water (50% WGC) during the last hydrothermal reaction exhibited superior characteristics compared to electrodes synthesized using 0, 25, 75, and 100% DMF solutions.
Our electrode can efficiently produce hydrogen under a wide range of pH conditions, making it versatile and adaptable for various practical applications. It is a step towards sustainable and efficient hydrogen production, which is essential for a cleaner energy future.
Wee-Jun Ong, Supervisor and Associate Professor, School of Energy and Chemical Engineering, Xiamen University
The 50% WGC electrocatalyst surpassed the platinum benchmark electrocatalyst, 20% Pt-C/CC, in the hydrogen evolution reaction (HER) under both acidic and basic conditions. To be more specific, the 50% WGC showed a lower overpotential, indicating less energy required for water splitting compared to the 20% Pt-C/CC. The overpotential for 50% WGC was 21.13 mV, while it was 46.03 mV for 20% Pt-C/CC.
The research team emphasizes that the development of cost-effective and energy-efficient electrocatalysts, such as the 50% WGC catalyst, is of utmost importance in advancing global clean energy objectives.
We aim to explore the scalability and practical implementation of our self-supported electrode technology. Our ultimate goal is to contribute to the transition to a sustainable energy landscape, where hydrogen can play a crucial role as a clean and renewable energy source.
Wee-Jun Ong, Supervisor and Associate Professor, School of Energy and Chemical Engineering, Xiamen University
Jian Yiing Loh, affiliated with the School of Energy and Chemical Engineering and the Center of Excellence for NaNo Energy & Catalysis Technology (CONNECT) at Xiamen University Malaysia in Selangor Darul Ehsan, Malaysia, also played a significant role in this study.
This research aligns with Malaysia's national policies, particularly the National Energy Transition Roadmap (NETR) and the Hydrogen Economy and Technology Roadmap (HETR), which aim to promote sustainable energy development in Malaysia over the next five years.
The research was funded by the Ministry of Higher Education (MOHE) Malaysia under the Fundamental Research Grant Scheme (FRGS) (Ref no: FRGS/1/2020/TK0/XMU/02/1), the Ministry of Science, Technology and Innovation (MOSTI) Malaysia under the Strategic Research Fund (SRF-APP) (S.22015).
The National Natural Science Foundation of China (Ref no: 22202168), the Guangdong Basic and Applied Basic Research Foundation (Ref no: 2021A1515111019), the Xiamen University Malaysia Investigatorship Grant (Grant no: IENG/0038), Xiamen University Malaysia Research Fund (ICOE/0001, XMUMRF/2021-C8/IENG/0041 and XMUMRF/2019-C3/IENG/0013) and the Hengyuan International Sdn. Bhd. (Grant no: EENG/0003) supported the study
Journal Reference
Yap, F. M., et al. (2023). Synergistic integration of self-supported 1T/2H−WS2 and nitrogen-doped rGO on carbon cloth for pH-universal electrocatalytic hydrogen evolution. Nano Research. doi.org/10.1007/s12274-023-6118-8.