Hydrogen is a highly combustible gas that, if produced in an environmentally friendly manner, can help the world meet its clean energy goals. The main impediment to producing hydrogen gas from water is the high amount of energy required for electrolysis or separating water molecules into hydrogen gas (H2) and oxygen (O2).
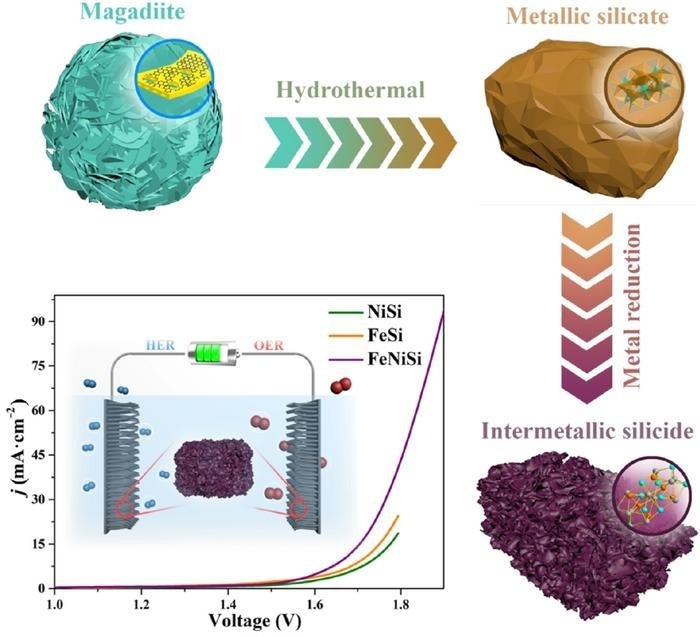 Natural clay magadiite containing silicon (Si) was heated in a sealed vessel in a water-based solution containing iron chloride (FeCl3) and nickel chloride (NiCl2) to create a metallic silicate made up of nickel (Ni), iron (Fe) and Si. The metallic silicate was then reduced by adding electrons to metallic silicate atoms with magnesium, salt and heat to create the more organized intermetallic silicide (ferric-nickel silicide) structure. The graph illustrates the lower voltage required for the ferric-nickel silicide (FeNiSi) alloy electrocatalyst to produce hydrogen and oxygen gas compared to NiSi and FeSi alloys. Image Credit: Nano Research Energy, Tsinghua University Press
Natural clay magadiite containing silicon (Si) was heated in a sealed vessel in a water-based solution containing iron chloride (FeCl3) and nickel chloride (NiCl2) to create a metallic silicate made up of nickel (Ni), iron (Fe) and Si. The metallic silicate was then reduced by adding electrons to metallic silicate atoms with magnesium, salt and heat to create the more organized intermetallic silicide (ferric-nickel silicide) structure. The graph illustrates the lower voltage required for the ferric-nickel silicide (FeNiSi) alloy electrocatalyst to produce hydrogen and oxygen gas compared to NiSi and FeSi alloys. Image Credit: Nano Research Energy, Tsinghua University Press
A novel electrocatalyst consisting of nickel (Ni), iron (Fe), and silicon (Si) that reduces the amount of energy required to synthesize H2 from water has been created in a simple and cost-effective way, enhancing the viability of H2 as a future clean and renewable energy.
Fossil fuels are the primary source of H2 produced today, which adds to global warming. A catalyst, or an agent that reduces the energy needed for a chemical reaction, is necessary to produce H2 from water through the hydrogen evolution process (HER). These catalysts, composed of rare earth metals like platinum until recently, decreased the viability and affordability of producing clean hydrogen.
An electrocatalyst, or catalyst that runs on electricity was created by a team of material scientists from the Dalian University of Technology in Dalian, China. They used low-cost materials and techniques to reduce the energy needed to produce pure H2 from water. Crucially, the ferric-nickel silicide (FeNiSi) alloy, or mixture, also lowers the energy needed to produce O2 from water, completing the bifunctionality of the catalyst.
Nano Research Energy published the findings on November 3rd, 2023.
What really limits the development and practical application of water electrolysis technology is electrocatalytic materials. At present, common catalysts, such as precious metals…, are mostly single-function catalysts, which limits the practical application of water electrolysis for hydrogen production. Therefore, the research and development of efficient, stable, cheap and environmentally friendly bifunctional electrocatalytic materials is a primary goal in the field of electrocatalysis.
Yifu Zhang, Study Senior Author and Researcher, School of Chemistry, Dalian University of Technology
These special compounds, known as transition metal silicide alloys, are widely utilized in energy-related industries, can be synthesized at low cost, and have prospective applications as water hydrolysis electrocatalysts.
Si atoms improve the stability, heat resistance, and accessibility of alloy transition metal atoms when electricity is applied. Transition metals are good catalysts that freely donate and take electrons in chemical reactions. These alloys are made from transition metals.
Two transition metals that work well in a transition metal silicide for water splitting are Fe and Ni.
Zhang added, “Nickel silicide has been deeply studied for its low resistance and high metal activity, especially in electrochemical fields. In addition, many recent studies have shown that Fe-Ni based materials have considerable potential in the field of electrochemical water splitting. The aim of this work was to develop a low-cost, environmentally friendly route to prepare iron nickel silicide as a bifunctional electrolytic water catalyst (EWS).”
The FeNiSi was created in two processes by the research team. First, natural clay magadiite, a silicon, iron, and nickel source, was cooked under pressure to form a ferric-nickel silicate.
The ferric-nickel silicate was then mixed with magnesium and sodium chloride (table salt) before being heated to form the ordered structure of the FeNiSi alloy. This was the first time a metallic silicide alloy had been created employing this type of chemical reaction with metallic silicates as the reaction ingredient.
The manufacturing procedure developed many pore patterns in the final FeNiSi alloy, boosting its surface area and overall electrocatalytic performance according to electron microscopy and X-Ray characterization techniques.
At a current of 10 mAcm-2, the FeNiSi alloy reduces the potential required to divide oxygen and hydrogen from water by 308 mV for the oxygen evolution reaction (OER) and 386 mV for the HER. After 15 hours of use, the electrocatalyst also proved to be durable.
The production of clean hydrogen gas for future energy needs is anticipated to be aided by FeNiSi and other transition metal silicates, according to the research team.
“This work not only provides an easy method for the synthesis of intermetallic silicide with considerable porous structures but also allows the intermetallic silicide to be considered as a bifunctional electrocatalyst for EWS. Low-cost and efficient intermetallic silicide electrocatalysts will provide new opportunities for renewable energy conversion,” Zhang further added.
Additional contributors include Xuyang Jing, Yang Mu, Zhanming Gao and Xueying Dong from the School of Chemistry at Dalian University of Technology in Dalian, China; Changgong Meng from the School of Chemistry and the College of Environmental and Chemical Engineering at Dalian University of Technology; and Chi Huang from the College of Chemistry and Molecular Sciences at Wuhan University in Wuhan, China.
The Large Instrument and Equipment Open Foundation of Dalian University of Technology and the Natural Science Foundation of Liaoning Province (2023-MS-115) provided funding for this study.
Journal Reference:
Jing, X., et al. (2023) Intermetallic ferric nickel silicide alloy derived from magadiite by magnesiothermic reaction as bifunctional electrocatalyst for overall water splitting. Nano Research Energy. doi:10.26599/NRE.2023.9120104