One of the most widely used rechargeable battery technologies in the modern era is lithium-ion batteries. Lithium-cobalt oxides (LiCoO2) are frequently utilized in these batteries as the constituents of positive electrodes, also known as cathodes (the conductors by which electric current either enters or departs a substance).
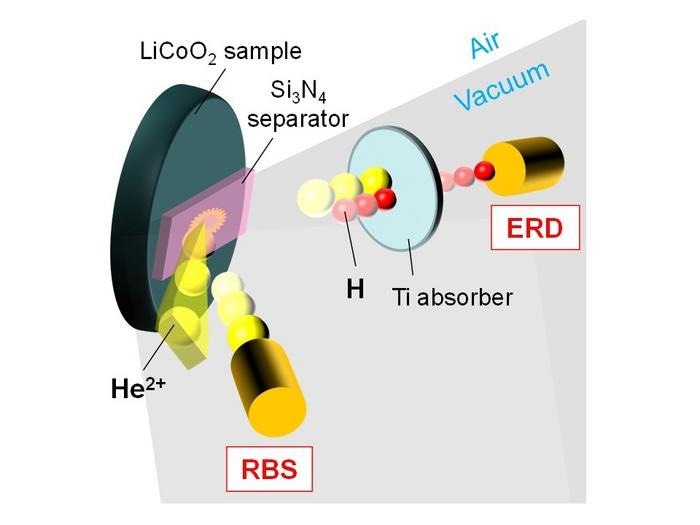
An oblique-view schematic image of simultaneous RBS and ERD analyses for a H2O-uptake LiCoO2 sample mounted on a sample holder in ambient air. Image Credit: Bun Tsuchiya
Lithium-ion batteries depend heavily on their cathode, which also affects the batteries’ capacity, endurance through several charge-discharge cycles, and thermal management capabilities.
The generation of hydrogen by the splitting of water is one of the main factors causing these batteries to deteriorate. Thus, understanding the accumulation and elimination of hydrogen in LiCoO2 can significantly improve the performance and longevity of solid-state lithium-ion batteries.
Furthermore, by using water splitting at ambient temperature, this information could give rise to novel methods of recycling spent lithium-ion batteries for the manufacture and storage of hydrogen.
Now, a team of researchers has thoroughly investigated the hydrogen uptake and loss in LiCoO2 cathode materials absorbed in water at room temperature. The study was led by Professor Bun Tsuchiya from the Department of General Education of the Faculty of Science and Technology at Meijo University and published in the International Journal of Hydrogen Energy on October 29th, 2023.
My motivation is to achieve the production of hydrogen (H2) through water (H2O) splitting at room temperature using certain oxide ceramic materials. Usually, H is dissociated from H2O at around 2000 K. However, this is too much energy for effective H2 fuel production and for solving current environmental problems, such as long-term carbon dioxide emissions.
Bun Tsuchiya, Professor, Department of General Education, Faculty of Science and Technology, Meijo University
The study aims to understand how LiCoO2 materials store and release hydrogen and determine the most stable sites within the LiCoO2 structure for trapping hydrogen. Numerous analytical approaches, such as weight increase and elastic recoil detection methods, were used to accomplish this. They discovered that when the material was submerged beneath the water for two minutes at particular temperatures, the quantity of hydrogen rose.
Furthermore, gas chromatography was employed to examine the hydrogen gas discharge and ascertain the temperature at which dissociation transpired, which was shown to be lower than 523 K.
Additionally, “density functional theory” calculations were performed as part of the study, and the results showed that hydrogen atoms extracted from water tended to choose lithium sites over other places in the LiCoO2 crystal structure.
Overall, the findings imply that LiCoO2 plays a major part in the process of water splitting to create hydrogen gas, which stores hydrogen at ambient temperature.
Tsuchiya envisioned, “If it becomes possible to make H2 from the inexhaustible H2O on earth with low energy input, I think that we can potentially establish a hydrogen-based society in the future.”
In conclusion, the study examined how hydrogen is stored and released in LiCoO2 cathode materials used in lithium-ion batteries. This work opens the door for the creation of more effective batteries and the low-energy production of hydrogen by water splitting, an ecologically benign method of energy storage! It does this by shedding light on a mechanism that causes deterioration in this widely used technology!
Journal Reference
Kataoka, K., et. al. (2024) Hydrogen absorption and desorption characteristics of H2O-uptake LiCoO2 materials at room temperature. International Journal of Hydrogen Energy. doi:10.1016/j.ijhydene.2023.10.039