In the study of chemistry, organic compounds can be anything from simple hydrocarbons to complex molecules with a variety of functional groups attached to the main carbon backbone.
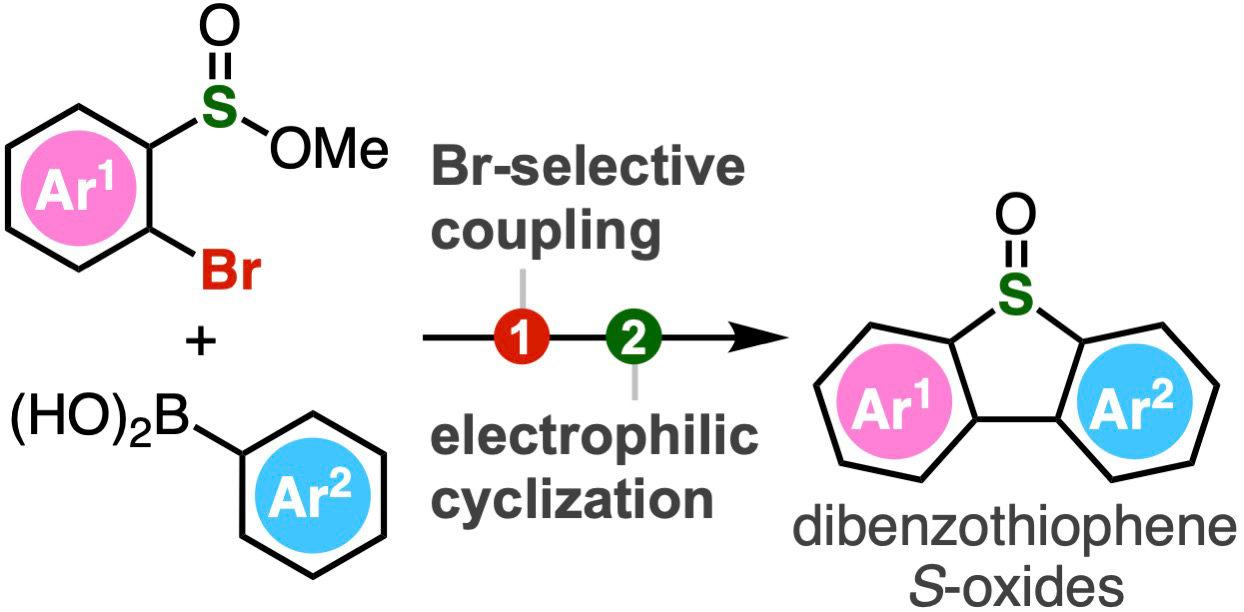
Schematic of the two-step process for producing dibenzothiophene S-oxides. A range of polysubstituted dibenzothiophene oxides can be synthesized through Br-selective coupling and subsequent cyclization by electrophilic activation. Image Credit: Suguru Yoshida from Tokyo University of Science
These functional groups give the compounds their unique chemical characteristics and take part in a number of chemical reactions, which makes them crucial building blocks for the synthesis of a wide range of compounds. As a result, scientists have been working hard to design new, extremely reactive functional groups for molecules.
Dibenzothiophenes and their derivatives with S-oxide or S,S-dioxide moieties—sulfur atoms bound to one or two oxygen atoms, respectively—are one such class of compounds. Chemical biology, materials chemistry, and pharmaceutical sciences are particularly interested in these compounds.
Dibenzothiophenes are made up of five-membered thiophene rings with four carbon atoms and one sulfur atom fused to benzene rings. Atomic oxygen is released by dibenzothiophene S-oxides in response to UV light.
This oxygen is helpful for cleaving DNA and oxidizing the enzyme adenosine-S'-phosphosulfate kinase, which is involved in various biological processes. Furthermore, by activating the S-O bond, various functional groups can be introduced, resulting in a vast array of molecules with a variety of characteristics and uses.
The traditional process of forming thiophene rings and then S-oxidation is used to produce functionalized dibenzothiophene S-oxides. Nonetheless, it is difficult to execute this response.
To tackle this, Tokyo University of Science (TUS) researchers Associate Professor Suguru Yoshida, Ms. Yukiko Kumagai, Mr. Akihiro Kobayashi, and Mr. Keisuke Nakamura created an easy two-step procedure for synthesizing dibenzothiophene S-oxides. The process entails the intramolecular electrophilic sulfinylation of 2-bromoaryl-substituted sulfinate esters coupled by Suzuki-Miyaura coupling.
The process’s specifics were revealed in the journal Chemical Communications, and the processes present opportunities for the synthesis of several significant sulfur-containing compounds in the biological sciences that have historically proven challenging to achieve with conventional techniques.
Dibenzothiophene oxides are attracting attention in the field of chemical biology, and several researchers have developed a reaction using dibenzothiophene oxide, which can now be synthesized using this method. We expect this research to elucidate life phenomena involving reactive oxygen species.
Dr. Suguru Yoshida, Associate Professor, Tokyo University of Science
A common organic reaction between boronic acids and organic halides that creates a new carbon-carbon bond is called the Suzuki-Miyaura coupling. The suggested procedure involves the first reaction of sulfinate esters with arylboronic acids in the presence of a palladium catalyst. Subsequently, Tf2O is used to activate the intermediate biaryl compounds, which triggers an electrophilic activation that cyclizes them.
This novel technique, created by Dr Yoshida and his team, can accommodate a wide range of functional groups, including highly reactive ones, making it possible to synthesize polysubstituted dibenzothiophene oxides that were previously unattainable.
This is in contrast to the traditional oxidation method of dibenzothiophene synthesis. By applying this technique, the scientists were able to create dibenzothiophene oxides with an o-silylaryl triflate moiety, which is a compound that can be used as an aryne generation site but is prone to damage when made with traditional methods.
The o-silylaryl triflate moiety can be converted into highly substituted arenes through a variety of processes, making it a useful reactive intermediate. Therefore, the suggested approach not only makes the synthesis process simpler but also makes a wide variety of dibenzothiophene S-oxides and derivatives possible.
Chemical biology has advanced significantly with the use of this novel approach. In the future, the researchers hope that these compounds will be useful in a variety of research fields, opening doors for new ideas and discoveries.
The proposed method can enable the synthesis of polysubstituted benzothiophene oxides, which are expected to be useful in a wide range of research fields.
Dr. Suguru Yoshida, Associate Professor, Tokyo University of Science
Journal Reference
Kumagai, Y., et al. (2024) Facile synthesis of dibenzothiophene S-oxides from sulfinate esters. Chemical Commununications.doi.org/10.1039/D3CC05703H