RIKEN researchers have identified an intriguing structure containing two water molecules that had previously been predicted but never observed. This discovery has the potential to have far-reaching ramifications, spanning from astrochemistry to metallic corrosion.
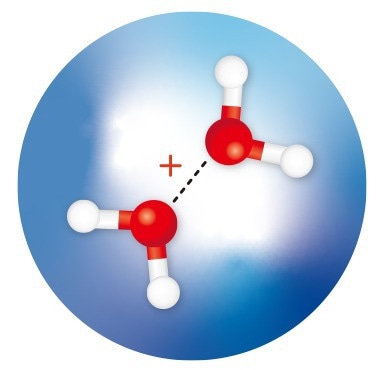 RIKEN researchers have isolated and observed water dimer cations (red spheres: oxygen atoms; white spheres: hydrogen atoms) within helium nanodroplets (large blue sphere) and determined the structures of the isomers. Adapted from Ref. 1. Image Credit: 2024 A. Iguchi et al.
RIKEN researchers have isolated and observed water dimer cations (red spheres: oxygen atoms; white spheres: hydrogen atoms) within helium nanodroplets (large blue sphere) and determined the structures of the isomers. Adapted from Ref. 1. Image Credit: 2024 A. Iguchi et al.
An energetic particle or photon can remove an electron from a water molecule, resulting in a positive ion (cation; H2O+) and an electron. The ionization of water can set off a chain reaction with adjacent molecules.
In addition to promoting corrosion at water-metal contacts, water ionization is crucial for radiation chemistry and biological activities. Therefore, one of the most important questions for physical chemists is how water ionization occurs.
According to calculations, two isomers of a positively charged ion of a water dimer—two water molecules with a weak connection between them—will quickly arise once a water molecule is ionized. A proton is transferred from one water molecule to another to generate one isomer (H3O+.OH) that has been seen.
Despite having a half-bond (or hemibonded) structure (H2O.OH2)+, the other isomer has never been isolated or verified by spectroscopic analysis. Its energy appears to be larger than that of the proton-transfer dimer, according to calculations.
By encasing both water dimer ions in small droplets of cryogenic helium, Susumu Kuma of the RIKEN Atomic, Molecular, and Optical Physics Laboratory and his colleagues have successfully isolated them. To ascertain their structures, they further employed infrared spectroscopy.
Kuma and colleagues synthesized the isomers in an ultracold environment. As helium atoms vanished from the droplet’s surface, the water molecules inside the helium droplets quickly cooled. This reaction quickly stabilized within the cool droplets, forming the metastable hemibonded isomer.
Next, Kuma and his colleagues used spectroscopic and computational techniques to investigate the co-existence of the two isomers. The molecular ions’ spectroscopic signals were nearly the same as those of bare ions—that is, ions without helium around them.
This finding indicates that we can directly compare measurements on the bare ions with results from quantum-chemical calculations. This greatly facilitates the structural analysis of the dimers.
Susumu Kuma, Research Associate, Okayama University
Kuma believes this result will help spur more research in this area.
Kuma added, “The discovery of the hemi-bonded water cations will promote further studies of primary events that are important for understanding the radiation chemistry of water.”
Kuma’s group plans to search for further structures that have not yet been noticed.
He concluded, “We plan to extend the size of the water complex cations in the helium droplets. We expect to find previously unobserved, but important, chemical structures in the spectra.”
Journal Reference:
Iguchi, A., et. al. (2024) Isolation and Infrared Spectroscopic Characterization of Hemibonded Water Dimer Cation in Superfluid Helium Nanodroplets. Small. doi:10.1021/acs.jpclett.3c02150.