Reviewed by Lexie CornerMar 15 2024
Despite their promise, fuel cells are costly due to high catalyst requirements to boost the activity of the oxygen reduction reaction (ORR). Researchers made a significant discovery when they discovered that caffeine could increase the ORR activity of platinum electrodes by 11 times. This research has the potential to improve fuel cell efficiency, decrease the need for extra platinum catalysts, and ultimately result in fuel cells that are both more affordable and efficient.
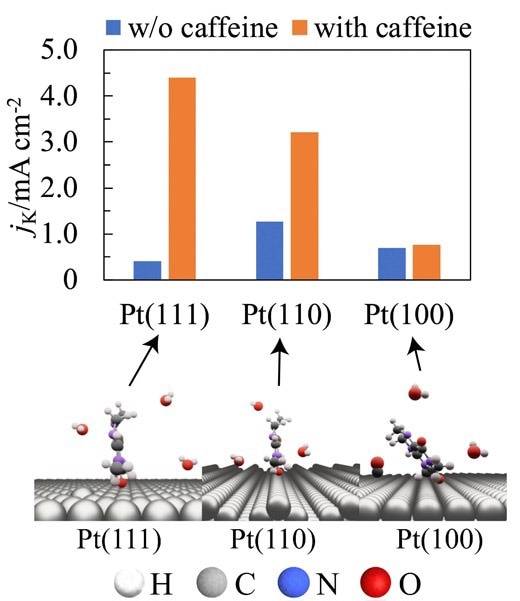 Adsorbed structure of caffeine on well-defined Pt single crystal electrodes and the activity of air electrode of fuel cell before (blue bar) and after (orange bar) caffeine modification. Image Credit: Professor Nagahiro Hoshi from Chiba University
Adsorbed structure of caffeine on well-defined Pt single crystal electrodes and the activity of air electrode of fuel cell before (blue bar) and after (orange bar) caffeine modification. Image Credit: Professor Nagahiro Hoshi from Chiba University
Fuel cells are a viable carbon-free energy source as the world looks to move away from fossil fuels. Fuel cells, which consist of an anode and a cathode divided by an electrolyte, directly transform fuel’s chemical energy into electricity. Fuel is delivered to the anode, and an oxidant—usually oxygen from the air—is added at the cathode.
At the anode of a hydrogen fuel cell, hydrogen is oxidized to produce hydrogen ions and electrons. Electricity is produced when the ions go from the electrolyte to the cathode and electrons pass through an external circuit. Water is the only byproduct of oxygen’s reaction with hydrogen ions and electrons at the cathode.
Water, however, impacts how well the fuel cell functions. It combines with the platinum (Pt) catalyst to generate a layer of PtOH on the electrode. This layer prevents the ORR from being catalyzed as effectively, resulting in energy losses. Fuel cells need a high Pt loading to operate efficiently, which substantially increases fuel cell expenses.
Now, Professor Nagahiro Hoshi, Masashi Nakamura, Ryuta Kubo, and Rui Suzuki from the Graduate School of Engineering at Chiba University, Japan, have discovered that adding caffeine to specific platinum electrodes can increase the activity of the ORR.
Their study was published in Communications Chemistry on February 3rd, 2024. This finding might lower the need for platinum, lowering the cost and increasing the efficiency of fuel cells.
Caffeine, one of the chemicals contained in coffee, enhances the activity of a fuel cell reaction 11-fold on a well-defined Pt electrode, of which atomic arrangement has a hexagonal structure.
Nagahiro Hoshi, Professor, Chiba University
To determine caffeine’s effect on the ORR, researchers monitored current flow via platinum electrodes submerged in a caffeine-containing electrolyte. The surface atoms of these platinum electrodes were organized in three distinct directions: (111), (110), and (100).
An increase in the concentration of caffeine in the electrolyte resulted in a significant improvement in the ORR activity of the electrode. When caffeine is present, it adsorbs onto the surface of the electrode and efficiently blocks the development of Pt oxide and hydrogen adsorption. Nevertheless, the way the platinum atoms were arranged on the electrode surface determined how the caffeine worked.
The ORR activity on Pt(111) and Pt(110) rose by 11 and 2.5 times, respectively, at a caffeine molar concentration of 1 × 10−6, whereas Pt(100) showed no discernible change. Using Infrared Reflection Absorption Spectroscopy, the researchers examined the molecular orientation of caffeine on the electrode surface to comprehend this discrepancy.
It was discovered that caffeine’s molecular plane is perpendicular to the Pt(111) and Pt(110) surfaces, where it is absorbed. Nevertheless, steric hindrances on Pt(100) result in its attachment with its molecular plane skewed with respect to the electrode surface.
Prof. Hoshi added, “The increased ORR activity of Pt(111) and Pt(110) was attributed to the decreased PtOH coverage and lower steric hindrance of the adsorbed caffeine. Conversely, for Pt(100), the effect of decreasing PtOH was counteracted by the steric hindrance of the adsorbed caffeine, and thus caffeine did not affect the ORR activity.”
Fuel cells have a longer lifespan than batteries. They can provide electricity for as long as fuel is available, making them useful for various applications such as structures, automobiles, and space missions. The suggested technique could enhance fuel cell designs and encourage their wider application.
The New Energy and Industrial Technology Development Organization (NEDO) 20001187-0 funded this study.
Journal Reference:
Hoshi, N., et. al. (2024) Enhanced oxygen reduction reaction on caffeine-modified platinum single-crystal electrodes. Communications Chemistry. doi:10.1038/s42004-024-01113-6