Reviewed by Lexie CornerMar 18 2024
Under the direction of Professor Wei Chen from the University of Science and Technology of China (USTC) of the Chinese Academy of Science (CAS), researchers have demonstrated the critical role that the halogen-mediated solvation structure plays in the de-solvation process of multivalent ions. The findings of the research were published in the journal Joule.
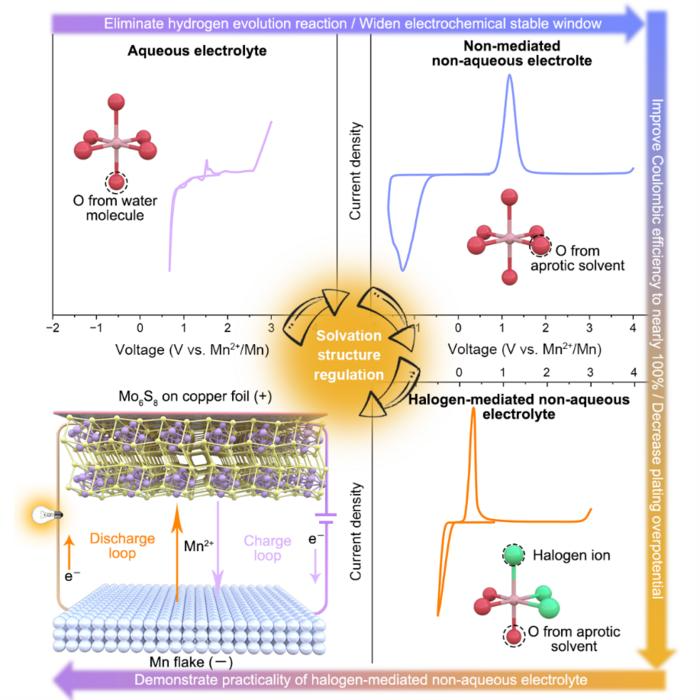 A novel electrolyte regulation strategy for multivalent metal batteries. Image Credit: Professor Chen’s team
A novel electrolyte regulation strategy for multivalent metal batteries. Image Credit: Professor Chen’s team
The researchers were able to fully demonstrate the significance of the halogen-mediated (with Cl as the primary research object) mechanism in lowering the overpotential of the multivalent metal ions deposition and enhancing the Coulombic and the dissolution/deposition efficiencies using manganese metal batteries (MnMBs) as the research platform.
Through both theoretical computations and experimental verification, the researchers were able to fully confirm that Cl played a full role in the solvation of Mn2+ in the designed electrolyte, converting the solvated structure of [Mn (Osol)6]2+ into [Mn (Osol)3Cl3]2+.
The solvated Mn-Cl bond is weaker than the Mn-O bond because Cl atoms have a larger radius and a lower charge density than other atoms. This lowers the deposition overpotential of the manganese metal anode, greatly reduces de-solvation energies during deposition, and significantly increases Coulombic and Faraday efficiencies.
Scientists put together symmetric and asymmetric cells to show the dependability of the halogen-mediated electrolyte. According to the experimental results, the electrolyte can sustain symmetric cell stable cycling for over 700 hours at a current density of 0.1 mA cm-2, which is significantly better than the stated manganese-metal battery electrolytes.
The symmetric cells had constant polarization values at various current densities, thus illustrating the electrolyte's superior multiplicity performance. The electrolyte demonstrated a deposition/dissolution overpotential of less than 200 mV and a Coulombic efficiency of about 100 %, even on various metallic or nonmetallic collectors.
When Ketjenblack (KB) is used as the current collector, an asymmetric cell made from this electrolyte can be steadily cycled for over 1000 hours.
Even at a larger surface capacity (5 mAh cm-2), the manganese deposition/dissolution efficiency remarkably held steady at 96.8 %.
The researchers also created a halogen-meditated non-aqueous manganese metal complete cell to further corroborate the efficacy of the intended halogen-mediated electrolyte. In the intended halogen-mediated electrolyte, the cell demonstrated an approximately 600-cycle stable cycling cycle, along with two distinct plateaus and a satisfactory multiplicity performance.
With the help of electrolyte engineering, the study provides a new avenue for the development of rechargeable non-aqueous MnMBs, which should help the electroplating and other multivalent metal battery sectors.
Journal Reference:
Shen, D., et al. (2024) A rechargeable, non-aueous manganese metal battery enabled by electrolyte regulation. Joule. doi.org/10.1016/j.joule.2024.01.012