In a recent paper published in the journal ACS Energy Letters, researchers from the Anhui Agricultural University and Guangxi University developed a semipermeable membrane that captures and transforms osmotic energy from salt gradients into electrical power.
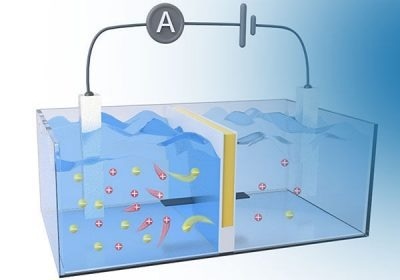
An improved membrane (yellow line) dramatically increased the amount of osmotic power harvested from salt gradients, like those found in estuaries where salt water (left tank) meets fresh water (right tank). Image Credit: ACS Energy Letters 2024.
Estuaries, where freshwater rivers merge with the salty sea, offer prime spots for birdwatching and kayaking. These unique environments, where waters of varying salinity mix, also hold potential for sustainable 'blue' osmotic energy production.
A study published in ACS Energy Letters reveals that researchers have developed a semipermeable membrane capable of harnessing this osmotic energy from salt gradients to generate electricity. In laboratory tests, the new membrane's output power density was more than double that of existing commercial products.
Osmotic energy, derived from salt gradients, has significant potential as a renewable energy source, although the technologies for harnessing it are still developing. One approach involves reverse electrodialysis (RED), where an array of membranes acts as a 'salt battery.' These membranes generate electricity from the pressure differences caused by the salt gradient. In this process, positively charged ions, such as sodium from seawater, migrate to the freshwater side, increasing the pressure on the membrane.
To enhance energy capture, the membrane must also maintain low internal electrical resistance, allowing electrons to flow freely in the opposite direction of the ions. Recognizing the need for improvements in ion flow and electron transport efficiency, researchers Dongdong Ye, Xingzhen Qin, and their team have developed a new semipermeable membrane. Made from environmentally friendly materials, this membrane is designed to minimize internal resistance and maximize power output.
The researchers developed a RED membrane prototype that featured separate channels for ion and electron transport, enhancing its efficiency. They achieved this by embedding a negatively charged cellulose hydrogel, responsible for ion transport, between layers of polyaniline, an organic conductive polymer used for electron transport.
Early tests validated their hypothesis: decoupled transport channels led to higher ion conductivity and lower resistivity than homogeneous membranes made from the same materials.
In a controlled experiment mimicking an estuary, the prototype displayed an output power density 2.34 times greater than that of a commercial RED membrane and sustained this performance over 16 continuous days underwater, proving its durability. Further demonstrating its utility, the team linked 20 of these membranes to form a 'salt battery' array, successfully powering a calculator, LED light, and stopwatch individually.
According to Ye, Qin, and their colleagues, their discoveries increase the variety of natural materials that can be utilized to create RED membranes and enhance the efficiency of osmotic energy harvesting, increasing the viability of these systems for practical application.
The authors received funding from the National Natural Science Foundation of China.
Journal Reference:
Xie, Z., et al. (2024) Decoupled Ionic and Electronic Pathways for Enhanced Osmotic Energy Harvesting. ACS Energy Letters. doi.org/10.1021/acsenergylett.4c00320