Reviewed by Lexie CornerMay 2 2024
According to a study published in ACS Materials Letters, researchers from Hokkaido University, Tohoku University, and Nagoya Institute, Japan, discovered an affordable and high-capacity cathode for next-generation lithium-ion batteries, with easily accessible mineral components that can significantly enhance its charge-recharge cycling.
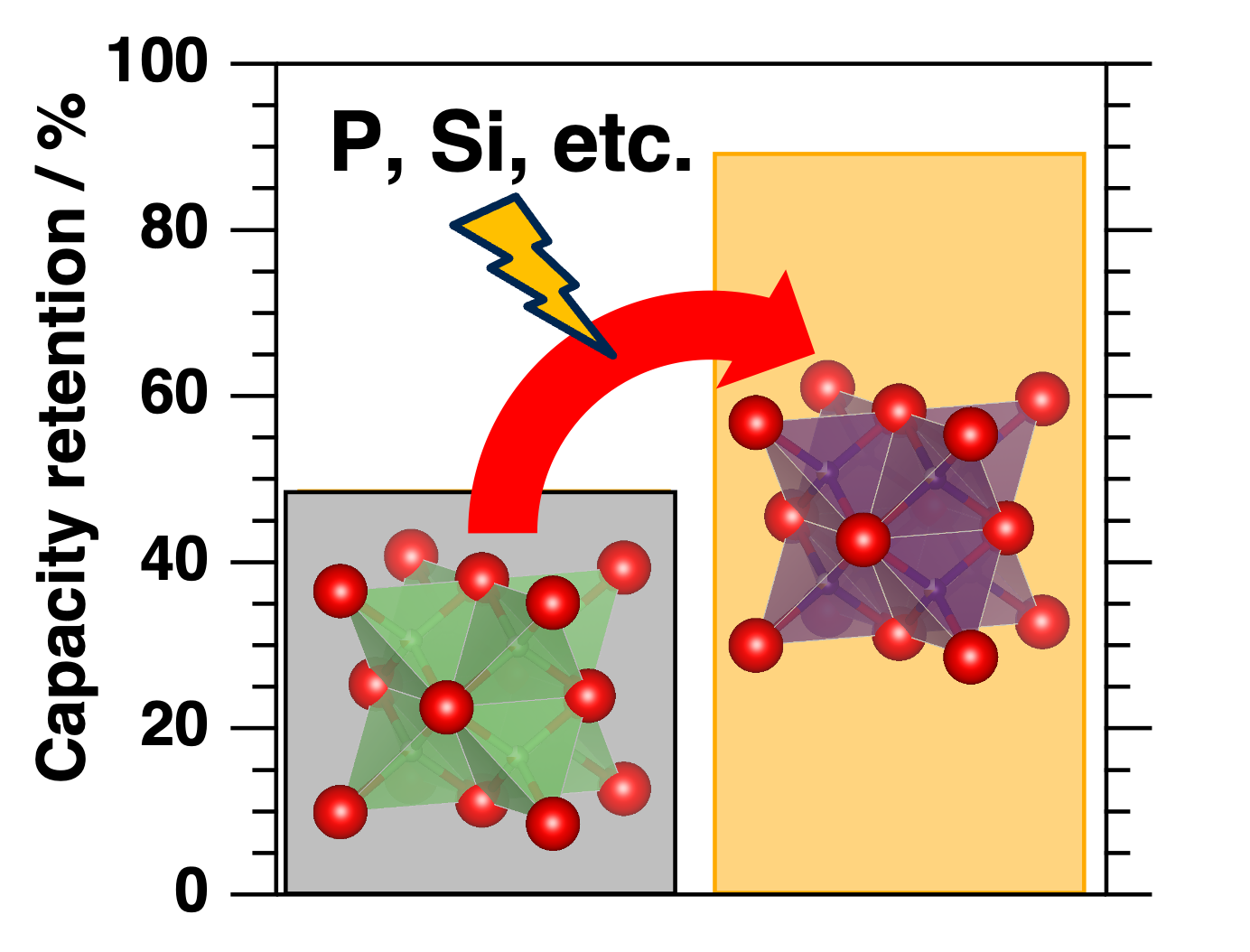 Capacity retention of the lithium-iron-oxide cathode is improved from 50 % to 90 % when doped with abundantly available elements such as aluminum, silicon, phosphorus, and sulfur. Image Credit: Hiroaki Kobayashi
Capacity retention of the lithium-iron-oxide cathode is improved from 50 % to 90 % when doped with abundantly available elements such as aluminum, silicon, phosphorus, and sulfur. Image Credit: Hiroaki Kobayashi
Adding small amounts of abundant elements enhances the energy capacity and charge-recharge cycling (cyclability) of lithium-iron-oxide, a cost-effective cathode material for rechargeable lithium-ion batteries.
Battery technology has become an essential part of modern life, with applications ranging from mobile phones to electric vehicles and large-scale power storage systems. Research efforts are ongoing to improve battery capacity, performance, and sustainability. One key challenge is reducing the use of rare and expensive resources in the battery cathode, where the key electron exchange processes take place.
The research goal was to enhance the cathode's performance using a specific lithium-iron-oxide compound. In 2023, a promising cathode material, Li5FeO4, with a high capacity exploiting iron and oxygen redox processes, was described. However, throughout the charging and recharging cycle, issues with oxygen generation arose during development.
We have now found that the cyclability could be significantly enhanced by doping small amounts of abundantly available elements such as aluminum, silicon, phosphorus, and sulfur into the cathode’s crystal structure.
Hiroaki Kobayashi, Associate Professor, Faculty of Science, Department of Chemistry, Hokkaido University
A major chemical feature of the increase was found to be the development of strong “covalent” interactions between the dopant and oxygen atoms inside the lattice. These bonds hold atoms together when electrons are shared between the atoms, rather than the “ionic” contact between positive and negatively charged ions.
The covalent bonding between the dopant and oxygen atoms makes the problematic release of oxygen less energetically favorable and, therefore, less likely to occur.
Hiroaki Kobayashi, Associate Professor, Faculty of Science, Department of Chemistry, Hokkaido University
To investigate the finer points of structural alterations in the cathode material brought about by the introduction of various dopant elements, the researchers employed theoretical computations and X-Ray absorption analysis.
This allowed them to offer theoretical justifications for the advancements they saw. Additionally, they demonstrated an increase in capacity retention from 50 % to 90 % by using electrochemical studies to evaluate changes in the cathode's energy capacity, stability, and cycling between the charging and discharging phases.
We will continue to develop these new insights, hoping to make a significant contribution to the advances in battery technology that will be crucial if electric power is to widely replace fossil fuel use, as required by global efforts to combat climate change.
Hiroaki Kobayashi, Associate Professor, Faculty of Science, Department of Chemistry, Hokkaido University
The next phase of the research will involve examining the challenges and opportunities associated with scaling up the methods to develop technology suitable for commercialization.
Journal Reference:
Kobayashi, H., et al. (2024) Toward Cost-Effective High-Energy Lithium-Ion Battery Cathodes: Covalent Bond Formation Empowers Solid-State Oxygen Redox in Antifluorite-Type Lithium-Rich Iron Oxide. ACS Materials Letters. doi.org/10.1021/acsmaterialslett.4c00268