Researchers at Doshisha University, under the direction of Professor Takuya Goto, have developed a practical way to create valuable hydrocarbons from CO2 in an effort to further current research on efficient electrochemical conversion. The study was released online on May 17th, 2024, and will be formally published in the journal Electrochimica Acta on July 20th, 2024.
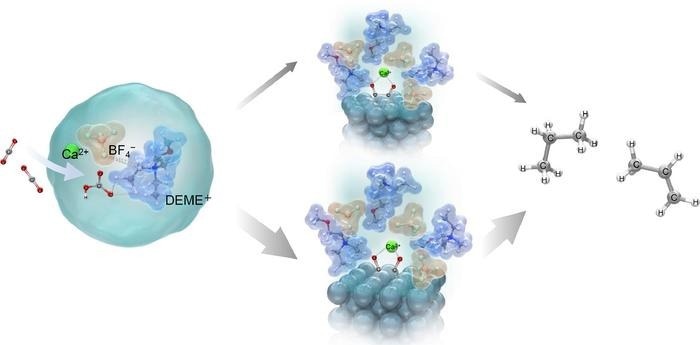 The production of hydrocarbons occurs through two intermediates formed on the surface of the silver electrode to produce useful hydrocarbons like ethylene, ethane, propylene, and propane. Image Credit: Takuya Goto from Doshisha University
The production of hydrocarbons occurs through two intermediates formed on the surface of the silver electrode to produce useful hydrocarbons like ethylene, ethane, propylene, and propane. Image Credit: Takuya Goto from Doshisha University
Electrochemical conversion of CO2 into fuel and chemicals using electricity offers a promising approach to reducing emissions. This process transforms carbon dioxide, captured from industrial activities and the atmosphere, into useful resources traditionally obtained from fossil fuels.
By utilizing captured CO2 in this way, we can decrease our reliance on non-renewable energy sources and create a sustainable loop of resource utilization, thereby reducing our environmental impact while simultaneously generating valuable products.
Using an ionic liquid containing metal hydroxides as the electrolyte, the research team—which also includes Dr. Yuta Suzuki from the Harris Science Research Institute and Ms. Saya Nozaki from the Graduate School of Science and Engineering—produced ethylene and propane on a basic silver (Ag) electrode.
Most studies on CO2 electrolysis with room-temperature liquid electrolyte have focused on the electrode’s catalytic properties. In this groundbreaking study, we focused on the electrolyte and succeeded in producing valuable hydrocarbon gas even on a simple metal electrode.
Takuya Goto, Professor, Doshisha University
Ionic liquids provide distinct benefits for the electrochemical reduction of CO2, functioning effectively across a wide voltage range without decomposing, while being non-flammable and possessing high boiling points. Their stability allows the electrolyte to endure the high temperatures produced during the exothermic reduction of CO2.
In their research, scientists explored the electrochemical conversion of CO2 and water using N, N-diethyl-N-methyl-N-(2-methoxyethyl) ammonium tetrafluoroborate (DEME-BF4) as the electrolyte. This specific electrolyte offers an ideal environment for CO2 reduction, with DEME+ ions increasing the solubility of CO2 and enhancing the participation of more CO2 molecules in the reaction. Additionally, its hydrophilic properties facilitate the supply of hydrogen ions necessary for converting CO2 to hydrocarbons when mixed with water.
The study found that the addition of aqueous solutions containing metal hydroxides, such as calcium hydroxide (Ca(OH)2), sodium hydroxide (NaOH), and cesium hydroxide (CsOH), to the electrolyte was shown to improve the electrochemical conversion of CO2 to hydrocarbons. These hydroxides react with CO2 to generate bicarbonates (HCO3−) and carbonates (CO32−), which increases CO2’s availability for use in electrochemical processes.
Performing room temperature electrolysis (298 K or 25 °C) in a CO2 atmosphere, the team successfully converted CO2 to ethylene (C2H4), ethane (C2H6), propylene (C3H6), and propane (C3H8), achieving the highest current efficiencies for these products using the DEME-BF4 electrolyte mixed with water and Ca(OH)2. The efficiencies reached up to 11.3 % for propane and 6.49 % for ethylene, surpassing those with other metal hydroxides by over 1000 times.
The high efficiency of this process was elucidated through Raman spectroscopy and density functional theory (DFT) calculations, which showed that bicarbonate ions formed from the interaction of CO2 with OH- ions in the electrolyte form a stable complex with DEME+ and BF4– ions [DEME+–BF4−-HCO3−-Ca2+].
CO2 and HCO3- species then adsorb onto the electrode surface as CO− ads species. These adsorbed ions strongly interact with Ca2+ ions in the electrolyte, forming two distinct intermediate structures: Structure A, with a Ca2+ ion coordinated with two CO− ions on three Ag atoms, and Structure B, where the Ca2+ ion is coordinated with two CO− ions on two Ag atoms. This interaction with Ca2+ ions is key as it stabilizes the adsorbed species, facilitating the subsequent electrochemical reactions.
The researchers suggest that structure B is more stable and is the preferred pathway for ethylene production, whereas structure A leads to the production of propane.
“We showed that tailoring the electrolyte can lead to molecular-level changes in the phase transformation of CO2 in bulk solution and at the electrode/ionic liquid electrolyte interface and proposed a process that enables the synthesis of unique hydrocarbons such as C3,” Prof. Goto added.
These results provide insight into the mechanisms, including the function of calcium ions, that are involved in the conversion of CO2 at the interface between ionic liquid-based electrolytes and metal electrodes. These discoveries can aid in the creation of electrolytes that effectively convert CO2 into useful hydrocarbons.
Prof. Goto concluded, “The physicochemical knowledge of this new route from CO2 decomposition to synthesizing useful hydrocarbons, as revealed in this study, will be instrumental in advancing CO2 utilization technology and contributing to academic progress in materials science.”
A research grant from The Iron and Steel Institute of Japan on steel carbon neutrality and JSPS KAKENHI Grant Number JP22K14700 provided partial funding for this study.
Journal Reference:
Nozaki, S., et. al. (2024) Electrochemical synthesis of C2 and C3 hydrocarbons from CO2 on an Ag electrode in DEME-BF4 containing H2O and metal hydroxides. Electrochimica Acta. doi:10.1016/j.electacta.2024.144431