In a recent study published in the journal Science, researchers from Argonne National Laboratory identified the cause of the decline in lithium-ion battery performance in nickel-rich cathodes. This discovery was largely attributed to the team's innovative analysis technique.
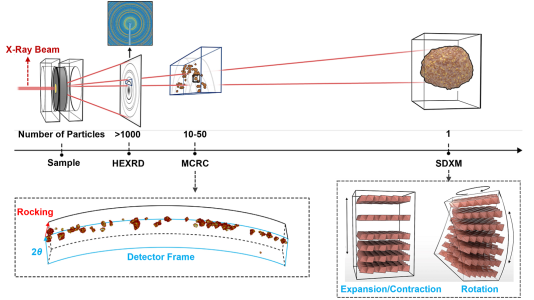
Method used for studying failure mechanisms in battery materials at sizes from 1 to 1000 particles. HEXRD = high energy X-Ray diffraction; MCRC = multi-crystal rocking curve; SDXM = scanning diffraction X-Ray microscopy. Lower right: lattice shape change with charge and discharge. Image Credit: Argonne National Laboratory
The first generation of lithium-ion batteries for electric vehicles has been a remarkable success story. However, the question remains: What changes to battery materials will drive further advancements to extend driving range and lower costs?
Intense research has focused on developing a better positive electrode, or cathode, for lithium-ion batteries. The cathode is a crucial component, and several potential materials promise significantly higher energy storage, leading to longer driving ranges. Unfortunately, the capacity, or the amount of current flowing out over a given time, tends to decline rapidly with charge-discharge cycling for reasons that are not yet fully understood.
Our method should also be useful to understanding failure mechanisms in other battery types than present-day lithium-ion.
Tongchao Liu, Assistant Chemist, Argonne National Laboratory
Researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have discovered the main reason why and how one of the more promising cathode materials degrades with use. This material is lithium nickel manganese cobalt (NMC) oxide, rich in nickel and in the form of single nanosized crystals. In single crystals, all the atoms are arranged in a highly ordered pattern.
Liu said, “Nickel-rich NMC is especially appealing because it uses 70-80% nickel, a high-capacity material, and requires much less cobalt.”
Cobalt is expensive and considered a critical mineral due to supply issues.
Typically, the nickel-rich NMC cathode consists of particles with multiple crystalline forms, or polycrystals, randomly oriented with respect to each other. During charge-discharge cycling, these clusters crack at the boundaries among the crystals, causing the cathode capacity to drop rapidly.
It was hypothesized that fabricating the cathode with single crystals instead of polycrystals would solve the cracking problem by eliminating the boundaries. However, even single-crystal cathodes failed prematurely, leaving scientists perplexed.
To uncover the mechanism, the team devised a pioneering method that combines multiscale X-ray diffraction and high-resolution electron microscopy. These material analyses were conducted at the Advanced Photon Source (APS) at Argonne, the National Synchrotron Light Source at DOE’s Brookhaven National Laboratory, and Argonne’s Center for Nanoscale Materials (CNM). All three are DOE Office of Science user facilities.
The problem with electron microscopy alone is that it only provides a snapshot of a small area on a single crystal. And while X-Ray diffraction offers insights into internal structures of many particles, it lacks surface-level information. Our method bridges this gap, offering a comprehensive understanding at the scale of one, 10 to 50, and 1,000 particles.
Tao Zhou, Materials Scientist, Center for Nanoscale Materials (CNM), Argonne National Laboratory
The atoms in single crystals are arranged in neatly ordered rows and columns called lattices. The team’s multifaceted analyses of single-crystal cathodes provided crucial information about changes in the lattice during charge and discharge cycles.
As Liu and Zhou explained, the introduction of a charge triggers a strain on the lattice, causing it to expand and rotate, disrupting the orderly pattern of atoms. Upon discharge, the lattice contracts to its original state, but the rotation remains. With repeated charge-discharge cycles, the rotation becomes more pronounced. This change in the cathode structure causes a steep performance drop.
Measurements taken with the Hard X-ray Nanoprobe, operated jointly by CNM and APS, were critical to gaining these insights.
The team’s new method was instrumental in understanding the burning issue of why nickel-rich NMC cathodes with single crystals fail so rapidly. This newfound understanding will give us ammunition to fix this issue and enable lower-cost electric vehicles with longer driving range.
Khalil Amine, Distinguished Fellow, Argonne National Laboratory
Liu said, “Our method should also be useful to understanding failure mechanisms in other battery types than present-day lithium-ion.”
Weiyuan Huang, Lei Yu, Jing Wang, Junxiang Liu, Tianyi Li, Rachid Amine, Xianghui Xiao, Mingyuan Ge, Lu Ma, Steven N. Ehrlich, Martin V. Holt, and Jianguo Wen are among the authors, in addition to Liu, Zhou, and Amine.
The DOE Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office provided support for this work.
Journal Reference:
Huang, W., et al. (2024) Unrecoverable lattice rotation governs structural degradation of single-crystalline cathodes. Science. doi.org/10.1126/science.ado1675