Researchers at Nanjing University of Posts & Telecommunications used a lithium-ion battery with fully stretchable parts, including an electrolyte layer that can expand by 5000%. Batteries, however, will require this ability to change shape to be used in flexible electronics, which are becoming increasingly popular for wearable health monitors. The study was published in ACS Energy Letters. The battery also maintains its charge storage capacity after almost 70 charge/discharge cycles.
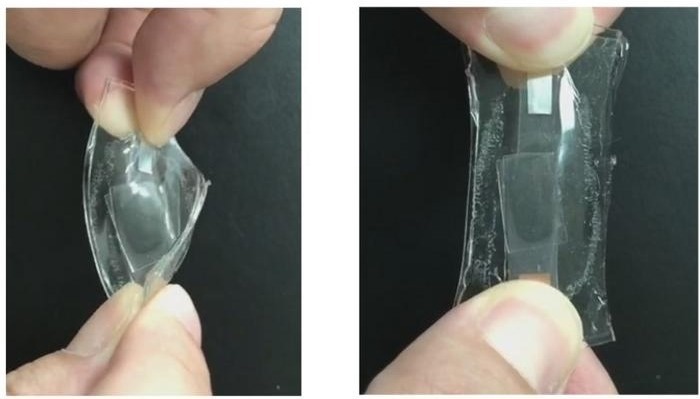 This lithium-ion battery has entirely stretchable components and stable charging and discharging capacity over time. Image Credit: Adapted from ACS Energy Letters 2024, DOI: 10.1021/acsenergylett.4c01254
This lithium-ion battery has entirely stretchable components and stable charging and discharging capacity over time. Image Credit: Adapted from ACS Energy Letters 2024, DOI: 10.1021/acsenergylett.4c01254
Batteries with comparable qualities are required for electronics that can stretch and bend. The majority of researchers who have tried to construct these batteries have done so using stiff components folded into origami-like shapes or woven conductive fabric. However, every component of a truly malleable battery needs to be elastic, including the middle electrolyte layer that balances charge and the electrodes that gather charge.
Currently, available prototypes for truly stretchable batteries have limited energy storage capacity, complicated assembly procedures, or only moderate elasticity—especially when repeatedly charged and discharged over time.
The latter may result from fluid electrolyte instability, which can move around when the battery changes shape or from a weak connection between the electrolyte layer and electrodes. Therefore, Wen-Yong Lai and colleagues aimed to create a fully solid, stretchable battery by fusing the electrolyte into a polymer layer sandwiched between two flexible electrode films instead of utilizing a liquid.
The group applied a thin layer of conductive paste containing carbon black, silver nanowires, and lithium-based cathode or anode materials onto a plate to create the electrodes for the fully elastic battery. On top of the paste was a layer of polydimethylsiloxane, a flexible substance frequently found in contact lenses. The researchers then added the ingredients for a stretchy polymer, a lithium salt, and a highly conductive liquid directly to this film.
These elements come together to create a solid, rubbery layer that can transport lithium ions and stretch to 5000% of its original length when activated by light. Ultimately, an additional electrode film was placed on top of the stack, and a protective coating was applied to the entire apparatus.
The new version of the solid stretchy battery had an average charge capacity that was approximately six times higher at a fast charging rate than that of a similar device with a traditional liquid electrolyte. The solid battery also ran through 67 cycles of charging and discharging with a more consistent capacity.
In other prototypes with solid electrodes, the capacity of the polymer electrolyte dropped by 1% in the first 30 cycles, while the liquid electrolyte's capacity dropped by 16% over a 1000-cycle period. Although there is still room for improvement, this new approach to producing fully stretchable, solid batteries may represent a significant advancement toward the development of wearable or implantable devices that can bend and move with the body.
The authors acknowledge funding from the following sources: China Postdoctoral Science Foundation, the Project of State Key Laboratory of Organic Electronics and Information Displays, NJUPT; the National Natural Science Foundation of China; and the Natural Science Foundation of Jiangsu Province.
The Foundation of Key Laboratory Flexible Electronics of Zhejiang Province; the Program for Jiangsu Specially-Appointed Professor; the NUPT "1311 Project" and Scientific Foundation; Jiangsu Province; and the Natural Science Foundation of NJUPT also funded the study.
Journal Reference:
Wang, S., et al. (2024) Elastic Polymer Electrolytes Integrated with In Situ Polymerization-Transferred Electrodes toward Stretchable Batteries. ACS Energy Letters. doi.org/10.1021/acsenergylett.4c01254