Reviewed by Lexie CornerSep 23 2024
In a study published in the Chinese Journal of Catalysis, a research team led by Prof. Gang Wu (University at Buffalo, State University of New York) and Dr. Dong Tian (University of Jinan, China) developed a self-supported catalyst with a 3D structure, composed of carbon nanotubes (CNTs) and Ni-Ni(OH)₂. The CNTs and heterostructure multifunctional components in the synthesized CNTs-Ni-Ni(OH)₂ catalyst synergistically enhance the hydrogen evolution reaction (HER), resulting in excellent catalytic performance.
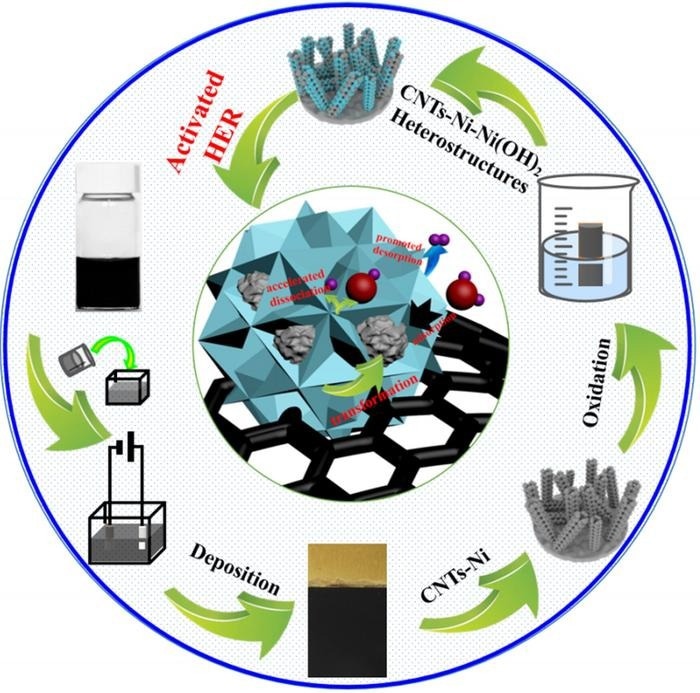 CNTs and Ni-Ni(OH)2 heterostructures were integrated onto a self-supported film by composite deposition and subsequent in-situ oxidation. The synthesized CNTs-Ni-Ni(OH)2 catalyst exhibited rather excellent performance for HER. The improved catalytic activity for HER could be attributed to the enhanced electrochemical active surface area and optimal adsorption strength of the H atom. The integrated CNTs and heterostructure could synergistically promote HER by facilitating water adsorption and dissociation as well as accelerating the desorption of Had. Image Credit: Chinese Journal of Catalysis
CNTs and Ni-Ni(OH)2 heterostructures were integrated onto a self-supported film by composite deposition and subsequent in-situ oxidation. The synthesized CNTs-Ni-Ni(OH)2 catalyst exhibited rather excellent performance for HER. The improved catalytic activity for HER could be attributed to the enhanced electrochemical active surface area and optimal adsorption strength of the H atom. The integrated CNTs and heterostructure could synergistically promote HER by facilitating water adsorption and dissociation as well as accelerating the desorption of Had. Image Credit: Chinese Journal of Catalysis
Combining renewable energy sources with the electrochemical dissociation of water allows hydrogen to store energy and potentially replace traditional fossil fuels. Ni-based catalysts, known for their high activity in HER, can reduce energy consumption, making them a cost-effective solution.
In alkaline electrolytes, HER involves the dissociation of adsorbed H₂O and the desorption of H₂, but these steps are typically slow on Ni surfaces. The incorporation of functional components like Ni(OH)₂ can significantly enhance the cleavage of the HO-H bond, while CNTs can influence the activation energy of catalytic reactions.
A novel approach is to develop a Ni-based catalyst with functional CNTs and a Ni-Ni(OH)₂ heterostructure to activate HER. The researchers designed a CNTs-Ni-Ni(OH)₂ hybrid film using composite electrodeposition followed by in-situ oxidation. The co-deposition of CNTs and Ni created a 3D CNTs-Ni hybrid film, and subsequent in-situ oxidation in H₂O₂ formed an interfacial Ni-Ni(OH)₂ heterostructure.
The synthesized CNTs-Ni-Ni(OH)₂ film exhibited outstanding catalytic properties for the HER in an alkaline solution, as demonstrated through linear sweep voltammetry and chronopotentiometry. The catalyst showed a 0 mV onset overpotential for HER, and overpotentials of only 65 mV and 109 mV at current densities of 10 mA/cm² and 50 mA/cm², respectively. Chronopotentiometry also revealed a low and stable overpotential, highlighting the catalyst's consistent performance.
The Ni-Ni(OH)₂ heterostructure and the integration of CNTs significantly enhanced the electrochemical active surface area. Additionally, the inclusion of CNTs and the heterostructure optimized the d band center of Ni (ɛd) to –1.179 eV, which is crucial for catalytic efficiency. The synergy between the CNTs and the heterostructure accelerated the HER process, reduced overpotential, and facilitated moderate hydrogen adsorption.
As a result, the developed 3D CNTs-Ni-Ni(OH)₂ catalyst showcased exceptional HER performance, providing a promising solution for HER in alkaline environments.
Journal Reference:
Zhao, W., et. al. (2024) Self-supported film catalyst integrated with multifunctional carbon nanotubes and Ni-Ni(OH)2 heterostructure for promoted hydrogen evolution. Chinese Journal of Catalysis. doi.org/10.1016/S1872-2067(24)60057-4