Reviewed by Lexie CornerSep 25 2024
Researchers at Osaka Metropolitan University have developed a new catalyst that efficiently converts a glycerol derivative into bio-based propylene, supporting the production of sustainable chemicals. Efficiently utilizing biomass from renewable sources is essential for achieving carbon neutrality. Glycerol, a major byproduct of biodiesel production, is a key feedstock in this process. The study was published in Chemical Communications.
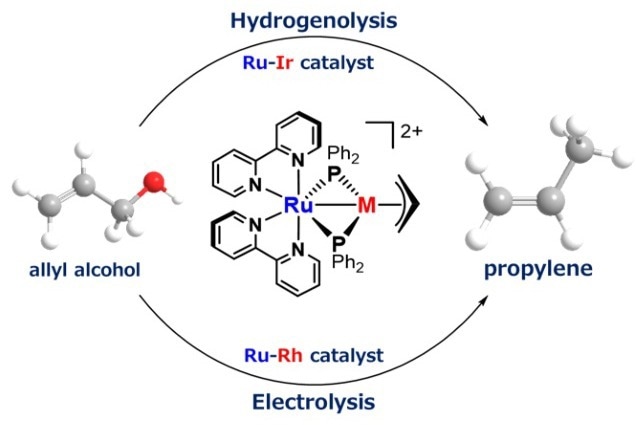 Turning glycerol component into propylene. A new catalyst can selectively reduce allyl alcohol, leading to a bio-based propylene. Image Credit: Osaka Metropolitan University
Turning glycerol component into propylene. A new catalyst can selectively reduce allyl alcohol, leading to a bio-based propylene. Image Credit: Osaka Metropolitan University
Propylene, commonly derived from petroleum, is widely used in the production of plastics such as food containers and car bumpers. To create bio-based propylene, the research team, under the direction of Associate Professor Shin Takemoto and Professor Hiroyuki Matsuzaka from the Graduate School of Science, created a catalyst that specifically breaks down the oxygen-carbon bond in allyl alcohol, a glycerol derivative.
This new catalyst enables the selective and efficient reduction of allyl alcohol to propylene using renewable energy sources like hydrogen or electricity. The catalyst features a unique molecule called a metalloligand, designed to facilitate the reversible binding of two metals within the catalyst. This property minimizes byproduct formation, maximizes selectivity, and enhances reaction efficiency.
Our research offers a sustainable alternative to conventional propylene production methods and can contribute to the development of an environmentally friendly chemical industry. We look forward to further advancing this technology and exploring its broader applications.
Shin Takemoto, Associate Professor, Graduate School of Science, Osaka Metropolitan University
Funding
Masuyakinen Basic Research Foundation and JSPS.
Journal Reference:
Kawaji, K., et al. (2024) Bimetallic Ru–Ir/Rh complexes for catalytic allyl alcohol reduction to propylene. Chemical Communications. doi.org/10.1039/d4cc01711k