Reviewed by Danielle Ellis, B.Sc.Sep 25 2024
Professors at the University of Science and Technology of China (USTC) have developed a novel strategy for solid-state electrocatalysis in lithium-ion batteries (LIBs). This approach breaks free from the conventional thinking that limits electrocatalysis to interfaces between liquid-solid and gas-solid. The study was published in the Journal of the American Chemical Society.
Schematics of solid-state electrocatalysis and related LIB performance. Image Credit: Prof. Ji’s team
Professors Hengxing Ji and Xiaojun Wu, along with Professor Xiangfeng Duan were involved in the study.
Anode materials like silicon and phosphorus, which store Li ions through alloying reactions, are commonly utilized in lithium-ion batteries (LIBs) because of their higher specific capacity when compared to traditional graphite anodes. The low lithiation kinetics in these anode materials, however, restrict LIBs' ability to charge quickly. Additionally, unlike in conventional electrocatalysis, the reactants and products of the Li-alloying reaction of anode materials are in solid phases. Research on electrocatalysis in solid-state reactions is, therefore, desperately needed.
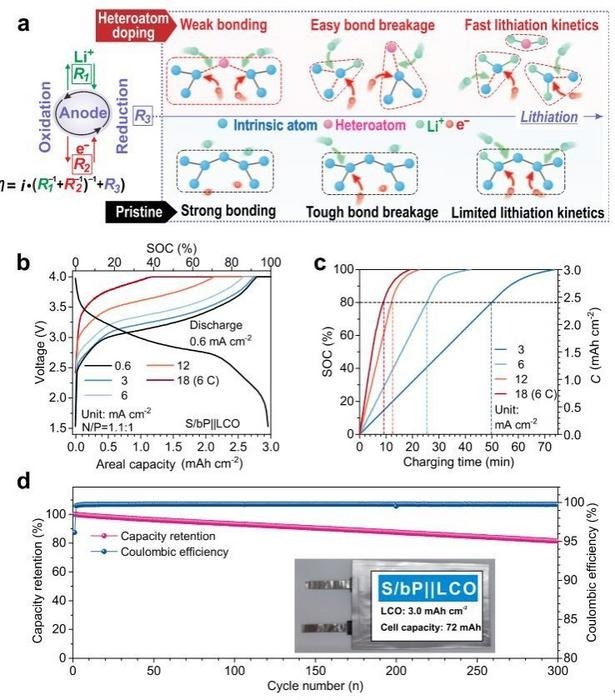
The team used heteroatom doping—boron for silicon and sulfur for phosphorus—to speed up Li-alloying reactions in solid-state electrode material to overcome this difficulty. The alloying reactions of anode materials can be facilitated by highly active sites provided by a critical heteroatom doping concentration of approximately 5%, as demonstrated by theoretical calculations and X-ray absorption spectroscopy (XAS), which in turn promotes intrinsic chemical bond breaking.
As a result of this bond cleavage at doped sites, the anode materials continually split into smaller unit cells, increasing the number of reactive sites and improving reaction dynamics.
Using this method, the group was able to develop an ultrafast-charging battery with a lithium cobalt oxide (LCO) cathode and a sulfur-doped black phosphorus (S/bP) anode. The battery surpassed previously published LIBs with an amazing performance, recharging 80% of its energy in 9 minutes with an energy density of 302 Wh kg-1. Additionally, this ultrafast charging performance held steady for over 300 cycles.
The study opens the door to electrocatalysis in solid-state reactions, a significant step toward the development of high-energy, fast-charging batteries with enormous industrial potential.
Journal Reference:
Zhou, E., et al. (2024) Solid-State Electrocatalysis in Heteroatom-Doped Alloy Anode Enables Ultrafast Charge Lithium-Ion Batteries. Journal of the American Chemical Society. doi.org/10.1021/jacs.4c03680.