AZoM talks to Dr. Daniel Abraham about his research surrounding lithium-ion batteries and the challenge of fast charging in particular.
Please can you introduce yourself, your background, and how you began researching your specialty of lithium-ion batteries?
I am a Senior Material Scientist at Argonne National Laboratory, a government research laboratory outside of Chicago, Illinois, USA. I started work at Argonne soon after completing my doctoral degree - more than 28 years ago! I have been researching lithium-ion batteries since 2001. Our team conducts mission-driven research to develop materials and methodologies to enhance the performance, safety, and life of batteries for various practical applications.
With increasing attention being paid to electric vehicle use, how essential are lithium-ion batteries to science and society more generally?
Whether we realize it or not, lithium-ion batteries are pervasive in everyday life. They have transformed the mobile phone and computer industry by making the devices lighter and smaller. They are set to refashion transportation as the automotive industry migrates from fossil-powered internal combustion engines to battery-powered electric motors to propel the vehicles. The batteries are being used to store energy generated by renewable sources, such as the sun, wind, and tides. Power tools, medical equipment, smartwatches, drones, and space satellites - all of these contain lithium-ion batteries.
Could you briefly describe how lithium-ion batteries operate, and the role graphite plays in their functioning?
A lithium-ion battery contains four basic components: a positive electrode (cathode), a negative electrode (anode), an electrolyte that allows lithium ions to flow between them, and a separator that physically separates the electrodes to prevent short circuits.
Most commercial lithium-ion batteries have lithium-containing nickel-cobalt-manganese oxide particles in the cathode and graphite particles in the anode. When you charge the battery, the current from the electrical outlet forces lithium ions to move from the cathode to the anode. This converts electrical energy from the outlet into stored chemical energy. When you unplug the battery and begin to use it, the lithium ions flow back to the cathode; the stored chemical energy is converted into a stream of electrons to power the device.
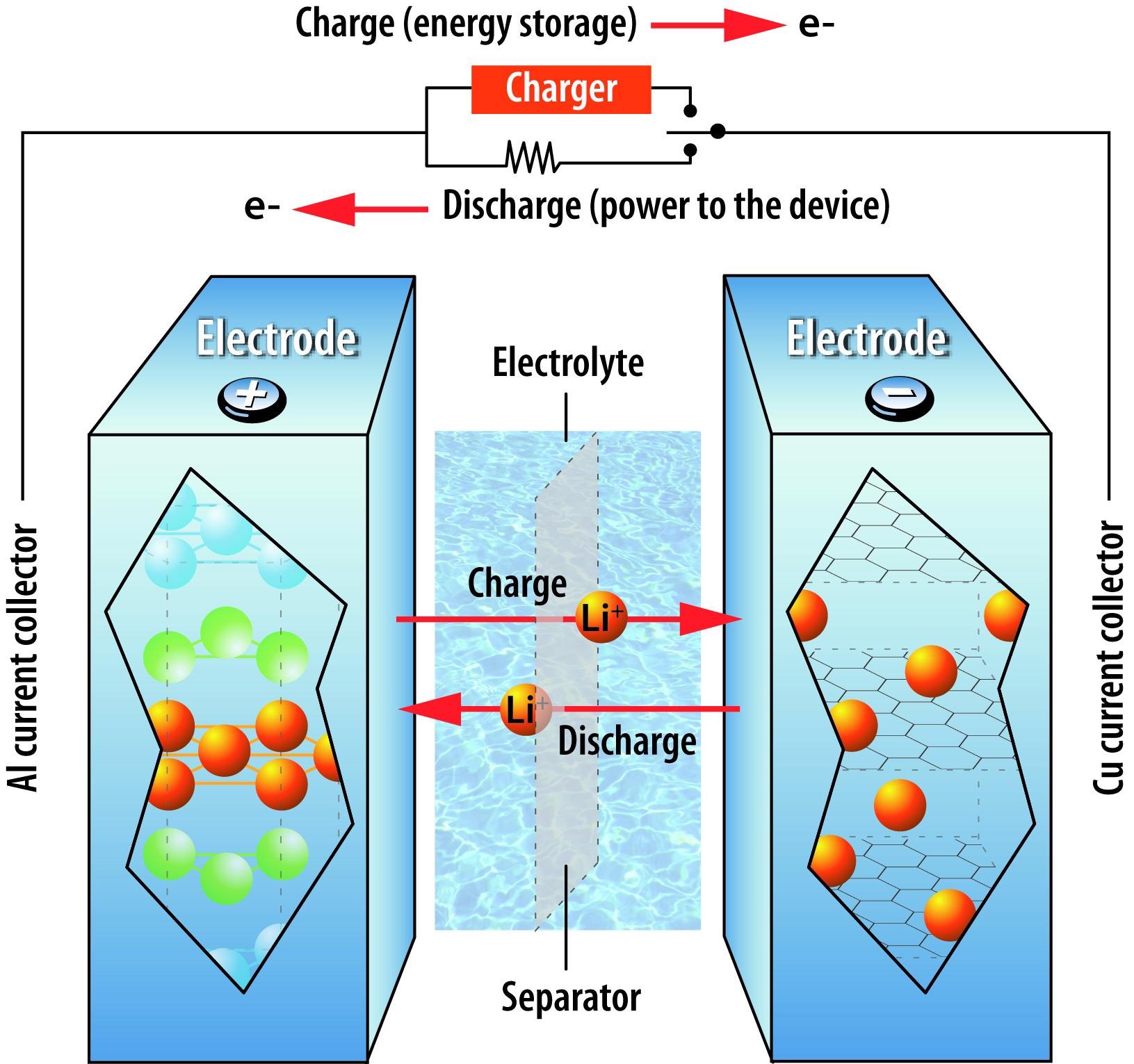
Image Credit: Daniel Abraham, Argonne National Laboratory
Your research considers the process of intercalation. Could you please describe in detail what this is and why it is significant?
Intercalation is the insertion of ions or molecules into solid materials with a layered structure; deintercalation is the reverse, wherein the ions or molecules are extracted from the material structure. Lithium-ion batteries rely on reversible intercalation and deintercalation processes.
During charging, lithium ions are deintercalated (extracted) from the oxide particles in the cathode and intercalated (inserted) between the carbon layers of graphite in the anode. The reverse occurs during discharge. Occasionally, instead of intercalation, the lithium-ions are involved in chemical reactions that trap and immobilize them, taking them out of circulation: this loss of mobile lithium ions decreases battery performance and life.
How is this process affected by charging a lithium-ion battery quickly? Were there any other further negative effects as a result of fast charging?
During slow charge, the lithium ions are gradually inserted between sheets of graphite. However, when the charge rates increase, instead of intercalation the lithium ions accumulate at the graphite, sticking to surfaces and even forming metallic lithium on the particles. This metallic lithium reacts with the surrounding electrolyte, becoming immobilized in chemical compounds that block the smooth movement of lithium ions into the electrode bulk. In addition, the rapid movement of lithium ions through the battery stresses the graphite, warping and distorting its atomic structure and affecting its ability to accept lithium ions and thereby degrading cell performance.
Could you describe the methods you used in order to study lithium plating and its effects on anodes?
We used a combination of microscopy, spectroscopy, and diffraction methods to examine the effect of fast charging. At the micrometer scale, we used scanning electron microscopy and energy-dispersive X-ray spectroscopy to show that the anode thickness increases because of lithium-containing reaction products that accumulate in the electrode pores. At the atomic scale, we used high-resolution electron microscopy and scanning electron nanodiffraction to reveal that the previously-layered atomic structure of graphite becomes increasingly warped because of repeated fast charging.
How can the negative effects incurred by fast charging be avoided or mitigated?
We are working on several approaches to enable fast charging, which include altering the graphite particle structures to enable faster intercalation and aligning electrode pores to quicken lithium diffusion in the anode. Additional approaches include designing electrolytes with high lithium-ion conductivity, using high-porosity separators that enable faster ion movement, and developing charging methods that prevent damage to the oxide and graphite structures.

Image Credit: buffaloboy/Shutterstock.com
Electric vehicles will become increasingly essential as we try to minimize our collective carbon footprint. How significant do you believe your research could be towards improving EVs?
Fast charging would ease consumer concerns regarding refueling time and accelerate the transition to electric transportation. However, charging at high rates can degrade battery performance, decreasing its range, life, and safety characteristics. Our research examines the mechanisms responsible for this loss of performance and recommends solutions for the design of batteries that can endure repeated fast-charging while reliably delivering high-performance over the lifespan of electric vehicles.
Was there any part of this research that was especially surprising or interesting to you?
We were surprised to find out that only a small number of fast-charge cycles is sufficient to induce significant and permanent disorder in graphite domains, especially in those particles closest to the electrolyte. Furthermore, we noted that these structural changes are highly non-uniform across the particles, which appears to be a consequence of reaction heterogeneities caused by the high-rate cycling.
What are the next steps for this subject matter? Do you hope to conduct further research around lithium-ion batteries, and fast charging effects in particular?
We are examining every part of the battery – cathode, anode, separator, and electrolyte – to gain insights that will lead to a device in which every component responds rapidly during charging and allows lithium-ion flow with minimal barriers and interruptions. Our recent studies have shown that the distribution of lithium-ion concentration across the electrodes is non-uniform during fast charging. Our present challenge is to design materials and electrodes that will store maximum charge as uniformly as possible. This will improve battery performance and improve the mileage delivered during fast charge.
Where can readers find more information?
More information on our research can be found at the following links:
About Dr. Daniel Abraham
 Dr. Daniel P. Abraham (Argonne National Laboratory) conducts research on lithium batteries used in electric vehicles, consumer electronics and grid energy storage. He has authored over 180 articles in peer-reviewed journals and delivered over 350 technical presentations in popular, academic, and industrial settings. His research areas include fast charging, crystal structure transformations in electrode materials, silicon electrode development, solid electrolyte interphase (SEI) formation/dissolution mechanisms, electrode stress evolution, electrode and particle coatings, electrolyte additives, and electrochemical modeling. His work enables the development of materials and components that enhance battery performance, life, and safety. Dr. Abraham is also a research advisor and mentor to various undergraduate students, graduate students, postdoctoral associates and junior scientists. He has received the Outstanding Postdoctoral Supervisor Award and the Pinnacle of Education Award for “exceptional work in developing the next generation of scientists and engineers.”
Dr. Daniel P. Abraham (Argonne National Laboratory) conducts research on lithium batteries used in electric vehicles, consumer electronics and grid energy storage. He has authored over 180 articles in peer-reviewed journals and delivered over 350 technical presentations in popular, academic, and industrial settings. His research areas include fast charging, crystal structure transformations in electrode materials, silicon electrode development, solid electrolyte interphase (SEI) formation/dissolution mechanisms, electrode stress evolution, electrode and particle coatings, electrolyte additives, and electrochemical modeling. His work enables the development of materials and components that enhance battery performance, life, and safety. Dr. Abraham is also a research advisor and mentor to various undergraduate students, graduate students, postdoctoral associates and junior scientists. He has received the Outstanding Postdoctoral Supervisor Award and the Pinnacle of Education Award for “exceptional work in developing the next generation of scientists and engineers.”
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.