Reviewed by Lexie CornerSep 26 2024
The Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) has published new research in Nature Nanotechnology highlighting manganese, the fifth most abundant metal in the Earth's crust, as a potential low-cost and safe alternative to lithium-ion batteries.
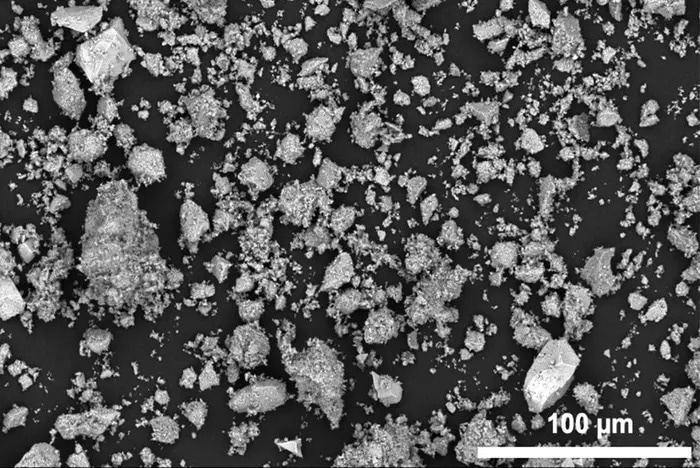 A new process for manganese-based battery materials lets researchers use larger particles, imaged here by a scanning electron microscope. Image Credit: Han-Ming Hau/Berkeley Lab and UC Berkeley
A new process for manganese-based battery materials lets researchers use larger particles, imaged here by a scanning electron microscope. Image Credit: Han-Ming Hau/Berkeley Lab and UC Berkeley
The use of rechargeable lithium-ion batteries is growing rapidly, powering everything from electric vehicles and energy storage systems to devices like laptops and smartphones. However, the common cathode materials, nickel and cobalt, are in limited supply.
Researchers have highlighted the potential of manganese in new cathode materials called disordered rock salts (DRX). Previous studies suggested that DRX materials needed to be ground into energy-intensive nanoparticles to function effectively.
The new research, however, shows that manganese-based cathodes can perform well even with particles roughly a thousand times larger than previously thought.
There are many ways to generate power with renewable energy, but the importance lies in how you store it. By applying our new approach, we can use a material that is both earth-abundant and low-cost, and that takes less energy and time to produce than some commercialized Li-ion battery cathode materials. And it can store as much energy and work just as well.
Han-Ming Hau, Ph.D. Student and Researcher, Battery Technology, Ceder Group, Lawrence Berkeley National Laboratory
The researchers used a novel two-day process that involves removing lithium ions from the cathode material, followed by heating it to a low temperature of around 200 degrees Celsius. This method is significantly faster than the current process for manganese-based DRX materials, which requires over three weeks of treatment.
The team used advanced electron microscopes to capture atomic-scale images of the manganese-based material in action. They found that their process resulted in a nanoscale semi-ordered structure, which enhanced battery performance by increasing the material's ability to store and deliver energy more densely.
Additionally, the researchers employed various X-ray techniques to study how battery cycling causes chemical changes in manganese and oxygen on a larger scale. By exploring manganese's behavior at different scales, the team identified new approaches to developing manganese-based cathodes and gained valuable insights into nano-engineering future battery materials.
Hau added, “We now have a better understanding of the unique nanostructure of the material, and a synthesis process to cause this ‘phase change’ in the material that improves its electrochemical performance. It’s an important step that pushes this material closer to battery applications in the real world.”
This research utilized three DOE Office of Science user facilities: the Advanced Light Source and the Molecular Foundry (National Center for Electron Microscopy) at Berkeley Lab, and the National Synchrotron Light Source II at Brookhaven National Laboratory. The study was funded by the DOE's Office of Energy Efficiency and Renewable Energy and the Office of Science.
Journal Reference:
Hau, H., et al. (2024) Earth-abundant Li-ion cathode materials with nanoengineered microstructures. Nature Nanotechnology. doi.org/10.1038/s41565-024-01787-y