Reviewed by Lexie CornerOct 14 2024
In a study recently published in The Frontiers in Energy, researchers from Fuzhou University presented a novel approach to improving the efficiency of direct ammonia protonic ceramic fuel cells (DA-PCFCs). The team enhanced the electrochemical performance of these cells by adding a CeO2-supported Ni and Ru catalyst layer, marking a promising first step towards more environmentally friendly energy sources.
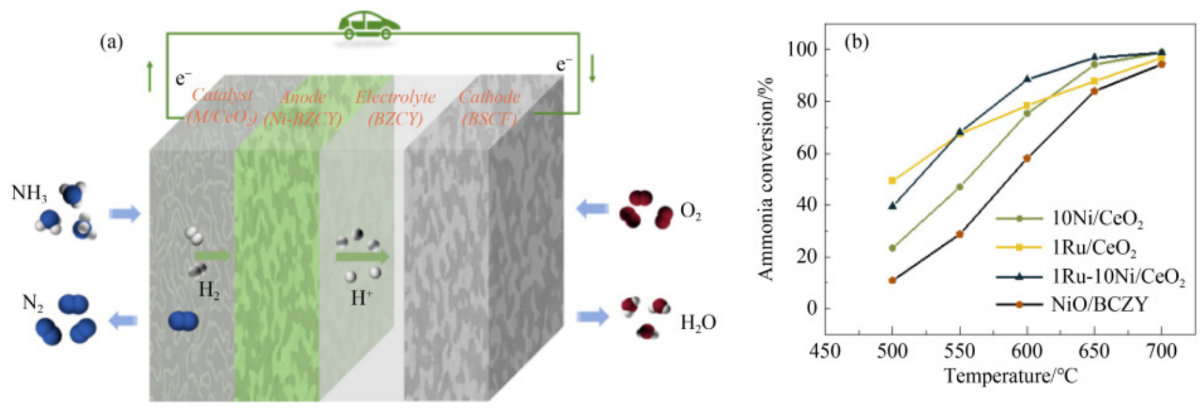 Ammonia conversion at different temperatures for the catalysts prepared. (a) Schematic diagram of modified direct ammonia protonic fuel cell; (b) comparison of catalysts in terms of ammonia decomposition rates (10Ni/CeO2, 1Ru/CeO2, and 1Ru-10Ni/CeO2). Image Credit: HIGHER EDUCATION PRESS
Ammonia conversion at different temperatures for the catalysts prepared. (a) Schematic diagram of modified direct ammonia protonic fuel cell; (b) comparison of catalysts in terms of ammonia decomposition rates (10Ni/CeO2, 1Ru/CeO2, and 1Ru-10Ni/CeO2). Image Credit: HIGHER EDUCATION PRESS
Due to its high hydrogen content and carbon neutrality, ammonia is becoming an attractive fuel for solid oxide fuel cells (SOFCs). However, its broader application has been hindered by the challenge of achieving adequate performance at intermediate temperatures (500–600 °C). The development of DA-PCFCs hinges on the creation of efficient catalysts that enhance ammonia breakdown and boost electrochemical processes.
A team from the Beijing Institute of Technology, Fuzhou University, and Qingyuan Innovation Laboratory, led by Yu Luo and Yunyun Huang, focused on developing a CeO2-supported catalyst layer to rebuild the anode surface of DA-PCFCs.
The researchers used BaZr0.1Ce0.7Y0.2O3–δ (BZCY) as the electrolyte and Ba0.5Sr0.5Co0.8Fe0.2O3–δ (BSCF) as the cathode to create an electrolyte-supported PCFC for their investigation.
They examined the performance of the PCFC utilizing NH3 as fuel within the working temperature range of 500–700 °C and compared it with conventional hydrogen fuel.
The electrochemical performance of the DA-PCFC was significantly enhanced by the addition of the M(Ni, Ru)/CeO2 catalyst layer. The degradation ratio of peak power densities (PPDs) of Ni/CeO2-loaded PCFC fueled with NH3 dropped at 700–500 °C when compared to H2 as fuel. At 700 °C, the decline was 13.3 %, and at 500 °C, it was 30.7 %.
The results showed that Ru-based catalysts are more promising for direct ammonia SOFCs (DA-SOFCs) at temperatures below 600 °C, while their effect is less significant above 600 °C compared to Ni-based catalysts.
The potential of CeO2-supported catalysts to improve the performance of DA-PCFCs is demonstrated in this study, which makes a substantial addition to the field of fuel cell technology. Enhancing electrochemical performance and lowering degradation rates at different temperatures present a feasible route to more sustainable and effective energy conversion systems.
In addition to addressing the technical difficulties with ammonia fuel cells, the research opens the door for the advancement and broader application of these green energy technologies.
Journal Reference:
Li, X., et al. (2024) Performance-enhanced direct ammonia protonic ceramic fuel cells using CeO2-supported Ni and Ru catalyst layer. Frontiers in Energy. doi.org/10.1007/s11708-024-0959-z.