An international team of researchers, headed by chemists from the University of Glasgow and battery testing specialists at Helmholtz Institute Ulm, used a material composed of chromium and selenium in a potassium-ion battery in a study that was published in the Journal of Materials Chemistry A.
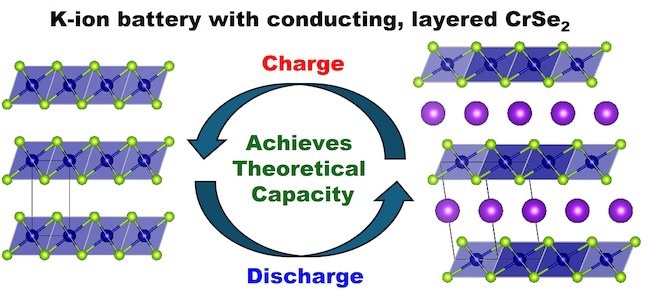
Image Credit: University of Glasgow
According to scientists, this advancement in material science could contribute to developing a new generation of reasonably priced batteries.
Thanks to potassium’s abundant availability and beneficial material qualities, such as quick charging, the discovery moves batteries closer to becoming a competitive substitute for lithium-ion systems.
According to experts, the batteries could eventually be cheaper and easier to produce than lithium-ion batteries, allowing for new uses such as storing renewable energy.
Lithium-ion batteries have become widely adopted in devices from smartphones to electric cars in recent years, and are capable of excellent performance, but lithium is a relatively rare, and therefore strategically important, element. Potassium is a much more abundant material, and potassium-ion batteries have a lot of potential as an alternative method of storing and delivering large amounts electricity. Adopting potassium-ion batteries for stationary storage purposes could help free up lithium resources for use in more energy-intensive mobile applications in the future.
Dr. Alexey Ganin, Study Lead Author and Senior Lecturer, University of Glasgow
Prussian White cathodes are used in some of the most effective potassium-ion battery designs. However, to achieve the best results, Prussian White must be mixed with carbon to increase its conductivity, complicating their design.
With less than 10% carbon, the researchers demonstrate in the study how their naturally conductive chromium selenide cathode achieves high performance. Their prototype’s 125 milliamp-hours per gram capacity is extremely near the device’s 127 milliamp-hours per gram maximum theoretical capacity.
Because of the material's layered structure, potassium ions can move more readily between the layers during charge and discharge. This enables even rapid charging and discharging of the battery while maintaining 85% of its capacity under laboratory conditions.
The team’s next task is to conduct more research to find an electrolyte that will improve battery performance in future battery design improvements.
Dr. Ganin added, “These are promising results, but we believe the performance of the battery could be boosted further with the right electrolyte. Designer lithium-ion battery electrolytes can be bought off the shelf, but further work is required to refine the performance of electrolytes for potassium-ion batteries. We’re keen to partner with robotics experts who can help us test the thousands of potential chemical combinations to find the best possible candidate for use in our battery.”
The study also included contributions from ICREA, the Catalan Institute of Nanoscience and Nanotechnology, the University of Kent, the Karlsruhe Institute of Technology, and Ulm University.
Journal Reference:
Li, W., et. al. (2024) Reversible K-ion intercalation in CrSe2 cathodes for potassium-ion batteries: combined operando PXRD and DFT studies. Journal of Materials Chemistry A. doi.org/10.1039/D4TA05114A