Scientists at Oregon State University have discovered a method to significantly enhance the carbon dioxide absorption capacity of a chemical structure, more than doubling its effectiveness for capturing emissions from factory flues.
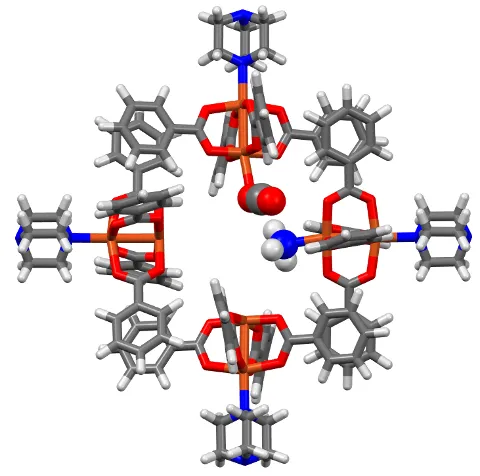 mCBMOF-1. Image Credit: Oregon State University
mCBMOF-1. Image Credit: Oregon State University
Research into metal-organic frameworks (MOFs) has uncovered a significant breakthrough in combating industrial greenhouse gas emissions. Industrial processes, including the burning of fossil fuels, are a major source of these emissions, with the Environmental Protection Agency estimating that 16 % of the United States' total carbon dioxide emissions come from industry.
The Oregon State team discovered a copper-based MOF whose carbon dioxide adsorption capacity more than doubled after being exposed to ammonia gas. This highlights the potential of MOFs as a powerful tool for reducing industrial carbon emissions.
The capture of CO2 is critical for meeting net-zero emission targets. MOFs have shown a lot of promise because of their porosity and their structural versatility.
Kyriakos Stylianou, Associate Professor, Oregon State University
MOFs are crystalline materials consisting of positively charged metal ions connected by organic linker molecules called ligands. These metal ions form nodes that bind to the linkers’ arms, creating a repeating cage-like structure with nanosized pores capable of adsorbing gases, much like a sponge.
MOFs can be tailored with a wide range of components, which define their properties. According to Stylianou, millions of potential MOFs exist, with over 100,000 already synthesized by chemists and the characteristics of hundreds of thousands more predicted.
Beyond capturing carbon dioxide and other gases, MOFs have diverse applications, including use as catalysts, in energy storage, drug delivery, and water purification.
In this study, the MOF known as mCBMOF-1 demonstrated a carbon dioxide uptake capacity on par with or exceeding that of traditional amine-based sorbents commonly used in industrial carbon capture. Additionally, MOFs offer significant advantages over amine-based sorbents, including greater stability and the ability to regenerate with less energy—achieved in this case by simply immersing the MOF in water.
The MOF is activated by removing water molecules to expose four closely positioned open copper sites. Then we introduce the ammonia gas, which causes one of the sites to be occupied by an ammonia molecule. The remaining sites attract CO2, promoting interaction with ammonia to form carbamate species.
Kyriakos Stylianou, Associate Professor, Oregon State University
Carbamates—compounds widely utilized in industry, agriculture, and medicine—are released during the water immersion process that regenerates the MOF's original structure, allowing it to be reused for continuous carbon capture.
According to Stylianou, these findings highlight the ability to tailor MOF structures with functional groups to improve their interactions with specific target molecules, such as carbon dioxide. This approach can also be adapted for other MOFs and different types of gases, broadening their potential applications.
Our study’s use of sequential pore functionalization to enhance CO2 uptake without significantly increasing regeneration energy is a terrific development. The formation of a copper-carbamic acid complex within the pores suggests strong and selective interactions with CO2, which is crucial for ensuring that CO2 is preferentially adsorbed over other gases in flue emissions.
Kyriakos Stylianou, Associate Professor, Oregon State University
Journal Reference:
Yadav, A. K., et al. (2024) Sequential Pore Functionalization in MOFs for Enhanced Carbon Dioxide Capture. JACS Au. doi.org/10.1021/jacsau.4c00808.