A research team led by Prof. Wei Chen at the University of Science and Technology of China (USTC) has developed a new chemical battery system that uses hydrogen gas as an anode. The study was published in the Angewandte Chemie International Edition.
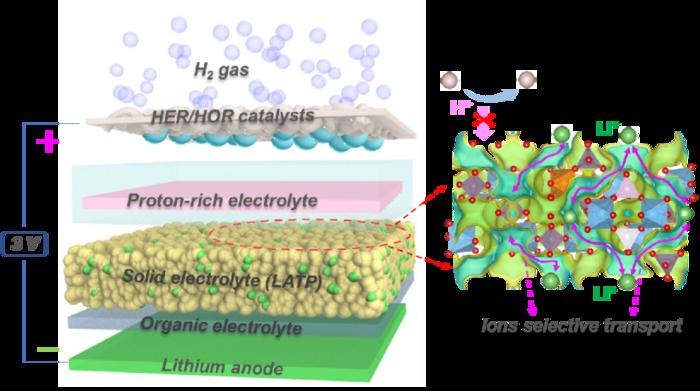 Schematic of the Li−H battery. (Image by USTC). Image Credit: CHEN Wei et al.
Schematic of the Li−H battery. (Image by USTC). Image Credit: CHEN Wei et al.
Hydrogen (H2) has gained popularity as a reliable and cost-effective renewable energy carrier due to its attractive electrochemical characteristics. However, classic hydrogen-based batteries predominantly use H2 as a cathode, limiting their voltage range to 0.8-1.4 V and overall energy storage capacity.
To address the limitation, the study team presented a revolutionary solution: use H2 as an anode to significantly boost energy density and working voltage. When lithium metal was used as the anode, the battery demonstrated remarkable electrochemical performance.
The researchers created a prototype Li-H battery system with a lithium metal anode, a platinum-coated gas diffusion layer functioning as the hydrogen cathode, and a solid electrolyte (Li1.3Al0.3Ti1.7(PO4)3, or LATP). This arrangement optimizes lithium ion transport while avoiding undesirable chemical interactions.
During testing, the Li-H battery achieved a theoretical energy density of 2825 Wh/kg while maintaining a stable voltage of approximately 3 V. It also achieved a remarkable round-trip efficiency (RTE) of 99.7%, demonstrating minimum energy loss during charging and discharging cycles while ensuring long-term stability.
To further improve cost-efficiency, safety, and manufacturing simplicity, the team created an anode-free Li-H battery that does not require pre-installed lithium metal. Instead, while charging, the battery deposits lithium in the electrolyte via lithium salts (LiH2PO4 and LiOH). The variant retains the advantages of the normal Li-H battery while adding new perks.
It allows for efficient lithium plating and stripping, with a Coulombic efficiency (CE) of 98.5%. Furthermore, it performs stably even at low hydrogen concentrations, decreasing the need for high-pressure H₂ storage. Computational modeling, such as Density Functional Theory (DFT) simulations, was used to better understand how lithium and hydrogen ions travel through the battery’s electrolyte.
This innovation in Li-H battery technology opens up new possibilities for sophisticated energy storage systems, including applications in renewable energy grids, electric vehicles, and even aerospace technology.
Compared to traditional nickel-hydrogen batteries, the Li-H system has higher energy density and efficiency, making it a promising contender for next-generation power storage. The anode-free variant paves the way for more affordable and scalable hydrogen-powered batteries.
Journal Reference:
Liu, Z. et. al. (2025) Rechargeable Lithium-Hydrogen Gas Batteries. Angewandte Chemie International Edition. doi.org/10.1002/ange.202419663