The scope of applications for biodegradable and biocompatible polymers – PLA and PLGA, is wide, from food packaging to farming materials to biomedical devices like stents, drug release systems, and absorbable surgical implants and threads. PLA is characterized by a low melt processing temperature, which makes it suitable for 3D-printing applications. Both PLGA and PLA are classified as ‘green’ polymers because the monomers which they are built from are produced using renewable sources. Certain properties like drug release rates or degradation can be altered to suit various applications by choosing different ratios, different molecular structures and molecular weights.
Samples that are used in manufacturing parts, degradation studies and material synthesis can be effectively monitored by GPC-SEC analysis. Acquiring information beyond mere numbers for molecular mass averages is possible by using advanced multi-detector GPC/SEC. Information rlating to the solution properties and structure can be acquired by combining data from light scattering, refractive index and viscometer detectors. This article describes the analysis of different PLA and PLGA polymer samples on the OMNISEC GPC/SEC system from Malvern Panalytical. This system incorporates all of the detectors described above.
Materials and Methods
Chromatographic Conditions
For the separation process, two LT5000L columns were used. Tetrahydrofuran (THF) was used as the mobile phase for the chromatographic system as well as the solvent for the samples. The columns, detectors and the Autosampler were maintained at 30 °C. A narrow polystyrene standard with a molecular weight of 104 Kg/mol was used for calibrating the system, and a broad polystyrene standard of known molecular weight was used for verifying the calibration. The concentrations of the prepared samples ranged from 1 to 5 mg/mL.
The OMNISEC SYSTEM
Modern GPC/SEC analyses use the OMNISEC SYSTEM as the standard. The system is divided into two modules: the OMNISEC RESOLVE, which is the chromatography module, and the OMNISEC REVEAL with a maximum of four detectors, which is the temperature controlled autosampler module.
The OMNISEC RESOLVE module consists of a pump, degasser, temperature controlled column compartment and the temperature controlled autosampler. The OMNISEC REVEAL consists of UV/VIS absorbance, differential refractive index, light scattering and viscometer detector. These modules together are an easy-to-operate, simple and robust package. The OMNISEC REVEAL can also be used as an add-on detector module in other chromatography systems.

Figure 1. The OMNISEC SYSTEM, with RESOLVE module on the left and REVEAL module on the right.
Results
A typical chromatogram showing the peak region and the plot of the intrinsic viscosity and derived molecular mass over the retention volume of a PLA sample measured using the OMNISEC system are shown in Figure 2.
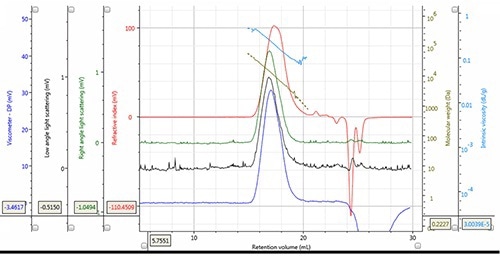
Figure 2. Chromatogram and derived data of PLA Mw = 12,188g/mol, Mn = 7,487g/mol, concentration 5.149mg/mL, 100µL injection volume.
Tetrahydrofuran is a common solvent that is used in numerous SEC-GPC applications and is also used for dissolving a number of PLA and PLGA samples. However, this sample-solvent combination exhibits a low refractive index increment (dn/dc) at concentrations of 0.045 to 0.051 mL/g.
The response of light scattering and refractive index detectors depends on the value of refractive index increment. By replacing the eluent with acetone, high signals for PLA and PLGA may be achieved, but this limits the use of the GPC-SEC system for other applications.
Although use of THF lowers the refractive index increase, its ability to assess a wide variety of samples makes it a preferred eluent for the characterization of PLA and PLGA on the OMNISEC REVEAL due to the improved sensitivity of the detector. Figures 2 and 3 illustrate the high quality of data achieved from both detector signals and the derived data for PLA and PLGA respectively.
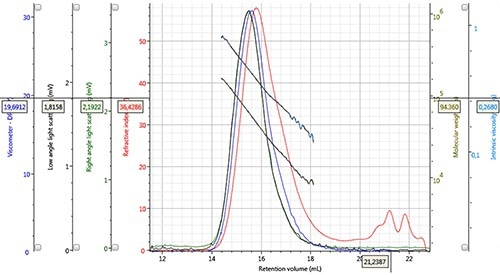
Figure 3. Chromatogram and derived data of PLGA 50:50, Mw = 44,628g/mol, Mn = 26,727g/mol, concentration 2.132mg/mL, 100µL injection volume.
Molecular Structure
Intrinsic viscosities across the peak can be determined with the help of the sensitive viscometer present in the OMINISEC REVEAL. The Mark-Houwink-plot (log η vs log MW) shows the structural variations in the samples.
The PLGA samples exhibit variations in composition due to marginal differences in coil density caused by compositional changes. The Mark-Houwink plot shown in Figure 4 represents duplicate injections of PLGA samples: two samples with varying molecular masses (50:50), and 65:35 and 75:25 compositions of lactide:glycolide. Based on the composition of the samples the Mark-Houwink plot can be divided into three areas: 75% lactide corresponding to the highest curve, 65% lactide at the middle of the curve and 50% lactide at the lowest point of the curve.
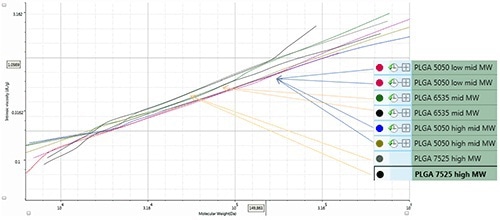
Figure 4. Mark-Houwink-plot for PLGA samples.
Reliable Data
A new type of autosampler suitable for handling the viscous sample solutions that are used in the GPS/-SEC analyzes is used in the OMNISEC RESOLVE. Reliable measurements are possible only through reliable injections; therefore, ten repeated injections of 100µL were made on the PLGA sample containing 50% lactic acid at a concentration of 2.132 mg/mL.
The measured parameters and the corresponding standard deviation (SD) values are listed in Table 1. It can be seen from the table that the recovery showed a relative SD value of 0.53% for the ten repeat injections. The recorded data demonstrated very good repeatability.
Table 1. Measured parameters and corresponding standard deviation values.
| Parameter |
Average value |
unit |
RSD / % |
| Mn |
26,102 |
g/mol |
2.32 |
| Mw |
44,722 |
g/mol |
0.37 |
| Mz |
67,009 |
g/mol |
1.22 |
| [η]w |
0.36 |
dL/g |
1.37 |
| Rh,w |
6.07 |
nm |
0.50 |
Conclusion
It is evident from the data that the OMNISEC GPC/SEC system is highly effective in the rapid and reliable determination of parameters like intrinsic viscosity, molecular mass, molecular structure and the hydrodynamic radius. Further, various compositions of PLGA can be distinguished distinctly based on the properties of the sample solutions. Only a reliable chromatographic method and a chromatographic system coupled with high performance detectors is capable of producing excellent reproducibility of results.
Due to the dependence of the final PLGA products on the parameters like molecular weight and PLGA composition, accurate measurement of these parameters will help understand and control the properties of the final PLGA products. For example, the release profile of drugs from a delivery implant or the degradation time of biodegradable sutures could be accurately controlled based on relevant measurements. The increase in need for precise control over drug delivery or other biomedical applications in turn increases the significance of such measurements.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.