Geologic storage of carbon is a potential method for reducing the anthropogenic emission of greenhouse gases. In this method atmospheric greenhouse-gas species, including CO2, are sequestered in underground reservoirs instead of being discharged into the atmosphere where they can eventually add to global climate change. However, the sequestration of CO2 has a number of challenges.
Soil gas monitoring at the sequestration site has to be done to ensure the site’s sustained integrity. Vibrational spectroscopic techniques like Raman spectroscopy and mid-infrared (IR) absorption provide a potential way to monitor critical species such as CH4, H4O vapor, O4, N4, CO, CO2, NOx, and SOx in soil gas to draw a comparison against ambient levels. This is done to evaluate the reliability of sequestration sites in real time.
Differentiating between variations in CO2 levels is considered to be the greatest problem in monitoring gases at sequestration sites. Such variations suggest an issue with the sequestration site as well as with those induced by natural phenomena, like respiration at plant roots, photosynthesis, and the natural cycling process of organic carbon in the soil.
At the sequestration site, the natural difference is calculated for a year or more to find out a baseline, and this is followed by making a comparative analysis of CO2 in the local atmosphere and within the borehole, as a part of the future monitoring of the sequestration site. The article shows a practical way of comparing the concentrations of CO2 at these sites.
Experimental Framework
Vibrational spectroscopy is suitable for investigating small homo- and heteronuclear soil gases. This method provides sharp and clear spectra that can be measured easily, and it is also capable of collecting spectra in real time by means of small sampling loops and similar types of standard instrumentation. Three compartments are included in this study’s experimental configuration: an external gas circulation module, a well completion (the finished well designed for injection), and a variety of sensors such as an IR analyzer, a Raman spectrometer, a pressure sensor, and moisture and temperature sensors. A synthetic diagram of the gas-analysis system is shown in Figure 1.
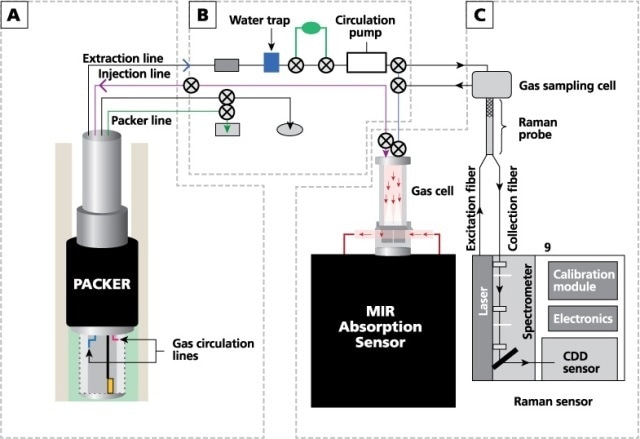
Figure 1. Schematic diagram of the gas-analysis system, showing (A) borehole and completion, (B) external module for gas circulation, and (C) IR and Raman analyzers. Adapted with permission from Ref. 1. © 2013 Elsevier.
Kaiser Optical Systems’ RamanRxn4™ gas-phase Raman analyzer was used to collect Raman spectra through a 532 nm laser excitation and with a working range of 175 to 4325 cm-1 for the Raman data. The gas circuit with a custom gas cell was mounted with an AirHead™ Raman probe head and linked to the spectrometer through optical fibers.
The custom gas cell was specifically designed to leverage multi-reflection scattering amplification by directing the laser via a sapphire window. Then with the help of mirrors, the laser is reflected prior to acquiring the Raman backscatter via the sapphire window and finally relaying it through the Raman probe head to the spectrometer.
Results
A standard Raman spectrum of soil gas at the sequestration site is shown in Figure 2. Main peaks for CO2, H4O vapor, O4, and N4 are marked.
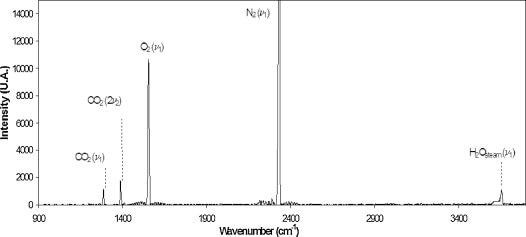
Figure 2. A typical Raman spectrum measured at the site in this study. Key peaks: H4O vapor (3657 cm-1), N4 (2331 cm-1), O4 (1555 cm-1), and CO2 (Fermi dyad at 1388 and 1285 cm-1). Reprinted with permission from Ref. 1. © 2013 Elsevier.
The Raman spectrum displays sharp, clear peaks, which enable quick and exact measurement of the various soil gases. During the injection operation, measurements of CO2 difference were compared over a period of four days in January 2011 (Figure 3), demonstrating that the Raman data relate well to those obtained from spectral measurements. Reference 1 includes the data from other soil gases (O4, N4, and H4O vapor).
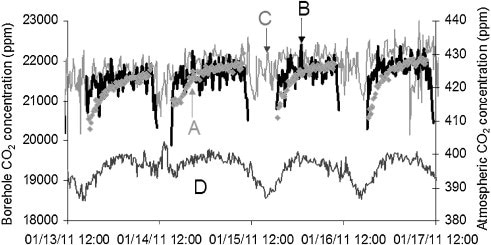
Figure 3. Diurnal variation of CO2 at the site and in the atmosphere over four days in January 2011. A, B are borehole CO2 concentrations calculated by IR, while C is the borehole CO2 concentration calculated from the Raman spectrum using the Fermi dyad at 1388 and 1285cm-1. D is the atmospheric CO2 concentration measured by IR. Reprinted with permission from Ref. 1. © 2013 Elsevier.
Conclusion
The study results show that Raman spectroscopy serves as a practical technique for monitoring soil gases at CO2 sequestration sites in real time. Combined with a well completion, a Raman spectrometer was effectively used to monitor the concentrations of a number of major soil gases at a sequestration site. Mid-IR absorption spectroscopy has a wide liquid water spectrum and cannot be applied in wet conditions. However, Raman spectroscopy is not sensitive to liquid water, it enables constant monitoring irrespective of dry or wet conditions. Conversely, as IR is highly sensitive in dry conditions, the combination of both Raman and IR allows each method to be utilized within its own suitable conditions.
In addition, fiber coupling allows the Raman probe head to be placed within the well completion, and the spectrometer base unit can be easily accessed in a surface module. To make a comparative analysis, a number of Raman probe heads can be set up at the following locations at the site:
- Natural sites such as fault zones and aquifers
- Boreholes
- Close to underground gas pipes, injection wells, and abandoned wells for which integrity happens to be a major concern

This information has been sourced, reviewed and adapted from materials provided by Kaiser Optical Systems, Inc.
For more information on this source, please visit Kaiser Optical Systems, Inc.