 Interview conducted by Mychealla RiceFeb 13 2018
Interview conducted by Mychealla RiceFeb 13 2018In this interview, Dr. Karen Esmonde-White, Senior Marcom Specialist from Kaiser Optical Systems Inc. talks to AZoM about using Raman as a real-time process tool for pharmaceutical manufacturing and bioprocessing.
What are the benefits of using Raman Spectroscopy as a technique over other optical techniques in sampling situations?
Raman spectroscopy is based on light scattering and does not rely on a specific path for the analytical light, giving it a tremendous advantage over other optical techniques when sampling solids or turbid media. Raman retains all the advantages of multiplexing and goes a step further in that the absence of a defined path length removes analytical constraints common to NIR and IR techniques.
Thus, multiple components can be measured at a single probe point. The spectrum is well defined on the wavelength axis with excellent peak separation, allowing the user to extract more information with less chemometrically intensive calibration. The excellent peak separation enables rapid method development and transferability across instruments and operating scales.
What are the benefits of incorporating Raman spectroscopy into existing processes to promote real-time measurements?
An in-line Raman measurement provides real-time process understanding, which enables in-process corrections and the ability to be integrated into advanced process control strategies. Customer examples show process Raman increases process efficiency, enables remote process monitoring and control, reduces contamination risk, and increases process robustness.
Moreover, the sharp bands of Raman spectra provide detailed chemical information on the process which enables the ability to transfer with a new material from R&D to manufacturing with excellent cross-scale model transferability.
Existing fiber-optic sampling probes can be integrated into a process, without needing to modify existing hardware, in a variety of installation locations. Installation examples of Raman include immersion probes in bioreactors, slip-streams, reaction vessels, or large volumetric probes in moving films, drug product manufacturing and continuous coaters.

The pharmaceutical processing industry often uses an active coating to coat tablets. How can Raman Spectroscopy measure this?
Raman spectroscopy is used throughout a pharmaceutical product’s lifecycle, from reaction monitoring of the active pharmaceutical ingredient (API) and its crystallization to manufacturing of the formulated drug product. Manufacturing of the formulated drug product is known as secondary processing.
Secondary processing is traditionally implemented as discrete steps, known as unit batch operations. Secondary processing can also be performed in a continuous mode where each of the drug product formulation steps are integrated into a continuous/flow operation. Some unit operations in secondary manufacturing include: granulation, blending, tableting and coating. Raman spectroscopy has been successfully integrated into unit batch and continuous operations in secondary processing.
Raman spectroscopy is well-suited for monitoring coating thickness while the tablets or capsule spheroids are being coated. For decorative coatings, the coating thickness may affect product bioavailability and storage stability. Active coatings contain API, and quantification of API within the coating mixture and measuring the coating’s thickness becomes a critical process parameter because it affects dosing.
A Raman probe using PhAT technology measures the API and the coating material in real-time within the process, so that the coating endpoint can be determined precisely and reproducibly. Several installation examples of a PhAT probe during a coating process can be seen in Figure 1. Raman can be used to monitor the disappearance of API peaks, the appearance of coating peaks, or both. A paper by Müller et al. demonstrates the value of PhAT technology in monitoring the endpoint of an active coating process.[1]
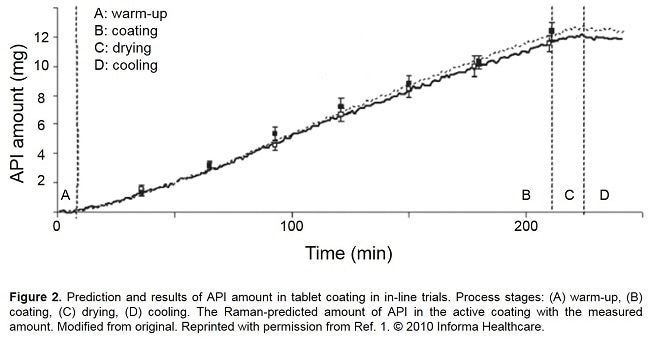
In their paper, Müller et al simulated an active coating process using a pan coater and a model API-coating system. Raman bands at 1290 cm-1 and 1330 cm-1 from the API were monitoring throughout the four coating process stages of warm-up, coating, drying and cooling, as shown in Figure 2. A recent paper by Barimani and Kleinbudde demonstrated successful Raman monitoring of tablet coating processes with colored coatings. [2]
How is Kaiser’s PhAT technology sampling useful for Raman-based process evaluation of tablets in the pharmaceutical industry?
The PhAT technology offers large volumetric Raman sampling and non-contact measurements. Large volumetric Raman uses a wide laser beam and multiple collection fibers to obtain data from surface and subsurfaces. These features ensure representative sampling when measuring processes that involve solids or turbid media.
An example of how a PhAT probe provided a more robust in-process measurement can be seen during wet granulation of nitrofurantoin. The wet granulation was performed in a high-shear mixer/granulator. The process was monitored by an immersion optic probe and a PhAT probe. Figure 3 contains the calibration plots for data from nitrofurantoin obtained with an immersion optic and for the PhAT probe.
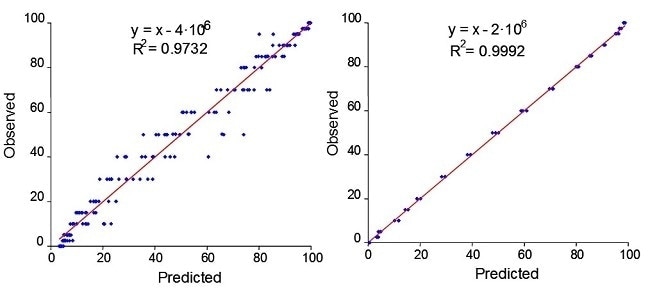
These results are very similar to those obtained for another model API wet granulation. Thus, the PhAT probe provides representative sampling that may improve model robustness in processes involving solids. Recent customer work has shown that measurements with a PhAT probe can be applied in a continuous solids manufacturing environment.
Please explain how Raman Spectroscopy can be used as a process analytical technology for pharmaceutical manufacturing and bioprocessing?
This topic does take up an entire paper! We encourage your readers to download the Esmonde-White et al open-access review article published in Analytical and Bioanalytical Chemistry.[3] Our review paper provides current industrial examples of Raman as a PAT and our perspectives on the future of Raman in process applications.
While the paper focuses on pharmaceutical manufacturing and bioprocessing, the principles of analysis can be applied to other fields such as polymer manufacturing, materials science, and chemical production.
What are the benefits of using Raman Spectroscopy as a real-time process analytical tool?
Raman is a proven technique in the world of process development. Process development is iterative, complex, and can take a long time if not properly controlled. There are numerous challenges as the process is scaled up from the laboratory to the pilot plant to manufacturing.
A significant challenge is to understand the physical and chemical properties of the process at each scale. Once a process is understood it can become optimized, effectively scaled, and properly controlled. There are multiple steps involved in understanding a process including: analyzing the process, understanding critical process parameters (CPPs) and what chemical or physical factors affect them, and controlling those process-dependent parameters.
Raman spectroscopy is uniquely suited for this task and has been proven to:
- Facilitate continuous processing / manufacturing approaches
- Provide robust cross-scale and cross-site method transferability
- Reduce cycle times
- Prevent reject product and waste
Scientifically and financially successful Raman applications have been demonstrated at all scales, from at line in the laboratory to on-line in manufacturing in PAC and PAT environments.
Emerging technologies such as transmission and enhanced reflection Raman have expanded the scope of Raman Spectroscopy in pharmaceutical manufacturing? What results can we expect to see?
New sampling technologies for Raman have expanded its capability even more. I think that we can expect to see Raman incorporated throughout a product’s lifecycle, from quality control of incoming raw material to real-time process/PAT to off-line quality assurance.

How can the RamanRxn System analyser from Kaiser Optical help with real time monitoring?
Raman spectroscopic analyzers are capable of precise process chemistry measurements with robust hardware that have lower maintenance demands and no consumables. Kaiser’s RamanRxn Systems™ family represents the state of the art in Raman analyzers for process applications.
All Kaiser systems share common technology features and allow simple transfer of analytical methods from research to process development to manufacturing.
From an engineering perspective, Kaiser has several features in its analyser software to enable its integration with 3rd party control systems and PAT Knowledge Management Solutions. Integration of any PAT into a control system is a personalized process that requires a customer to work closely with the analytical instrument vendor and a data management system, while considering the end user and plant environment.
As a result, the end user can run and control the Kaiser analyser using familiar control system tools without needing to learn instrument-specific software.
Where can our readers go to find out more?
We encourage your readers to visit the Kaiser website, and join us on YouTube, LinkedIn, Twitter and Facebook.
About Dr. Karen Esmonde-White
Dr. Karen Esmonde-White is a Senior Marcom Specialist at Kaiser Optical Systems Inc.

She earned her B.S. in chemistry from Wheeling Jesuit University, her M.S. in Chemistry, M.Eng. in Pharmaceutical Engineering and Ph.D. in Biomedical Engineering from the University of Michigan. Dr. Esmonde-White has over 6 years experience as an analytical chemist in the pharmaceutical industry.
Her research interests are in biological Raman spectroscopy, process analytics and instrument design.
1. Müller J, Knop K, Thies J, Uerpmann C, Kleinebudde P (2010) Feasibility of Raman spectroscopy as PAT tool in active coating. Drug Dev Ind Pharm 36:234–243 . doi: 10.3109/03639040903225109
2. Barimani S, Kleinebudde P (2018) Monitoring of tablet coating processes with colored coatings. Talanta 178:686–697 . doi: 10.1016/j.talanta.2017.10.008
3. Esmonde-White KA, Cuellar M, Uerpmann C, Lenain B, Lewis IR (2016) Raman spectroscopy as a process analytical technology for pharmaceutical manufacturing and bioprocessing. Anal Bioanal Chem 409:637–649 . doi: 10.1007/s00216-016-9824-1
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.